Discoloration and radiopacity of white mineral trioxide aggregate with various radiopacifiers
Abstract
The use of the mineral trioxide aggregate (MTA) has been expanded as the material can be applied on various treatment of endodontic care, which also has many advantages including bioactivity. Still, the discolorations of the materials have been presented as a concern related to esthetic appearances, which is caused by the presence of radiopacifiers. Therefore, the aim of this study was to investigate the color stability and radiopacity of white MTA with various radiopacifiers.
Four different radiopacifiers [bismuth oxide (BM), calcium tungstate (CT), barium oxide (BO), and zirconium oxide (ZO)] were used. The radiopacity was tested according to ISO 6876, and the color change before and after immersing in a 5% hypochlorite solution was tested using a spectrophotometer. The group with no radiopacifier (NR) was used as a negative control and ProRoot MTA (PR) was used as the commercial control. The immersion of the PR and BM in sodium hypochlorite resulted in a dark brown discoloration, in which the values were higher than the rest of the group (p<0.05). No change was observed in the NR. Moreover, the CT and ZO showed no color change compared to the NR (p>0.05). In terms of the radiopacity, the NR showed the lowest value as expected (p<0.05). Meanwhile, the BM showed the highest value (p<0.05), followed by PR and BO (p<0.05). The NR showed the lowest radiopacity values. The result of this study will be useful for future development of MTA that would have clinically adequate radiopacity with minimum discoloration.
초록
근관치료에 사용되는 Mineral Trioxide Aggregate (MTA)는 다양한 형태의 치과치료에서 사용될 수 있는 치과재료로 생체활성이 뛰어나다는 장점으로 최근 그 사용이 증가하고 있다. 하지만, MTA 중 특히 백색 계열의 MTA의 경우 성분에 포함되는 방사선불투과성 재료에 따라 변색이 일어날 수 있고, 이에 따른 심미적인 문제가 제기되어 왔다. 이에 본 연구에서는 총 4개의 방사선불투과성재료[bismuth oxide (BM), calcium tungstate (CT), barium oxide (BO), and zirconium oxide (ZO)]를 함유한 백색 MTA에 대한 변색과 방사선불투과성을 연구하였다. 방사선불투과성은 국제표준 ISO 6876에 따라 측정하였고, 변색은 5%의 NaOCl 용액에 침적 전과 후에 따른 색의 변화를 분광광도계로 측정하였다. 이 때 방사선불투과성 재료가 전혀 함유되지 않은 재료(NR)를 음성 대조군으로 사용하였으며, 시중에 판매되고 있는 ProRoot MTA (PR)를 판매 제품 대조군으로 설정하였다.
PR과 BM의 경우 NaOCl에 침적하였을 때 갈색으로의 변색이 관찰되었고 실제로 변색 또한 다른 시험군보다 높았다(p<0.05). 반면, NR의 경우에는 변색이 관찰되지 않았으며, CT와 ZO의 경우에도 NR과 비교하였을 때 변색 차이가 없었다(p>0.05). 방사선불투과성의 경우 예상했던 것과 같이 NR이 가장 낮았다(p<0.05). 반면, BM이 가장 높았으며(p<0.05) 그 다음에 PR과 BO가 다른 군에 비해 높았다(p<0.05). 본 연구 결과를 기반으로 추후 변색이 비교적 적으며, 임상적으로 충분한 방사선불투과성을 갖는 MTA의 연구가 가능할 것이다.
Keywords:
Discoloration, Mineral Trioxide Aggregate, Radiopacity키워드:
Mineral Trioxide Aggregate, 방사선불투과성, 변색Introduction
Mineral trioxide aggregate (MTA) is mainly composed of calcium silicate-based cement, Portland cement, and metallic oxide, which has been used for apexification, vital pulp therapy, root canal surgery, and perforation sealing (1-4).
In terms of the esthetic point of view, the discoloration of MTA has been reported as an inconvenient property for the earlier product, which may even cause a clinical misdiagnosis, and hence the color of MTA has been changed from gray to white. Still, the discoloration of MTA through various mechanisms has been reported(5-8).
The discoloration of both gray MTA (GMTA) and white MTA (WMTA) has been caused by metal oxides (iron, aluminum, and magnesium oxides) (9), blood contact (7), and the effect of light and oxygen (9, 10). The interaction of MTA with sodium hypochlorite (NaOCl) which is regularly used for irrigation in endodontics, resulted in a black MTA surface (6, 12, 13). Such incidence is common with MTA that uses the bismuth oxide as the radiopacifier, as it reacts with sodium hypochlorite, forming bismuth carbonate (13).
The sodium hypochlorite was used for root canal disinfection, smear layer removal, and the dissolution of pulp tissue, however it was reported to cause discoloration when coming into contact with WMTA (6, 13).
Materials and Methods
1. Materials
Bismuth oxide (Bi2O3, MW: 465.96, CAS No. 1304-76-3), calcium tungstate (CaWO4, MW: 287.392, CAS No. 7790-75-2), barium oxide (BaO, MW: 153.33, CAS No. 1304-28-5) and zirconium oxide (ZrO2, MW: 253.81, CAS No. 7553-56-2) were purchased from Sigma Aldrich (St. Louise, MO, USA). White Portland cement (Union Cement, Seoul, Korea) and calcium sulfate hemihydrates (Sam Woo Co. Ltd., Ulsan, Korea) were also purchased.
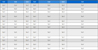
The radiopacifiers considered in this study are listed in Table 1. Each radiopacifier (20 wt%) was mixed with White Portland cement (75 wt%) and calcium sulfate hemihydrates (5 wt%). ProRoot MTA (PR, Dentsply, York, PA, USA) was used as a control product, and the other compositions are listed in Table 1(11). The test materials were mixed with a ball milling system using a zirconia ball and sieved. Then, each mixture was collected in a glass vial and stored in a desiccator before the tests. The material with no radiopacifier (NR) was used as the negative control.
2. Measurement of color change
Each material has been placed in a Teflon mold with a diameter of 15 mm and thickness of 2 mm. The hardened specimens were immersed in a 5% sodium hypochlorite solution for 24 h. The color changes before and after immersion in the sodium hypochlorite solution was measured using a spectrophotometer (CM-3500d, Minolta Co., Osaka, Japan) and digital camera (Canon IXUS, Singapore). The color change of each sample was calculated using the following formula:
3. Radiopacity test
According to ISO 6876 (14), each test material was mixed with distilled water and used to fill a mold (with a diameter of 10 mm and thickness of 1 mm) (14). The specimens were stored at 100% relative humidity in a 37℃ water bath for 24 h. The specimens were then placed on a digital radiographic sensor next to an aluminum step wedge and exposed to X-rays using an X-ray unit (Carestream CS7600, Siemens, Munich, Germany) operated at 70 kV and 10 mA, at a distance of 30 cm. The results were analyzed with Photoshop (Adobe, San Jose, CA, USA) by calculating the means of five sample points. The radiopacity in relation to thickness of aluminum step wedge has been calculated.
4. Statistics
The data were analyzed with a one-way ANOVA (PASW 18.0, IBM Co., Armonk, NY, USA) followed by Tukey’s statistical test. All the significance levels were fixed at 0.05.
Results
1. Color change
The color changes and images of the test specimens before and after immersion in the solution are shown in Figure 1. The PR, which contained bismuth oxide, showed the most significant color change, which was significantly higher than the rest of the samples (p<0.05). BM also showed a significantly higher color change than BO, CT, ZO, and NR (p<0.05). The BO changed to a light-gray color in which the change was significantly higher than CT, ZO, and NR (p<0.05). Finally, the CT and ZO showed no color changes as there were no significant difference in color changes between CT, ZO and NR (p>0.05).
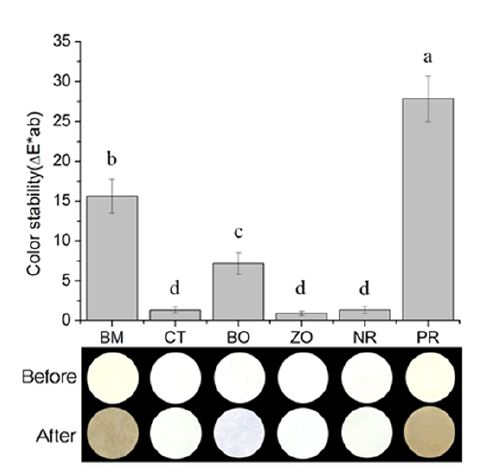
Color change before and after immersion in sodium hypochlorite solution. White MTA with four different radiopacifiers; bismuth oxide (BM), calcium tungstate (CT), barium oxide (BO), and zirconium oxide (ZO). The group with no radiopacifier (NR) was used as a negative control and ProRoot MTA (PR) was used as the commercial control. The same lowercase letters indicate no statistical significant differences (p>0.05).
2. Radiopacity
The mean radiopacity values (equivalent to thickness of Aluminum in mm, Almm) and images of the specimens are presented in Figure 2. The BM had the highest value compared to other samples (p<0.05). This was followed by BO = PR > CT > ZO > NR (p<0.05). All samples, except NR (which had no radiopacifier) showed radiopacity greater than 3 Almm, which is the radiopacity recommended by ISO 6876 (14).
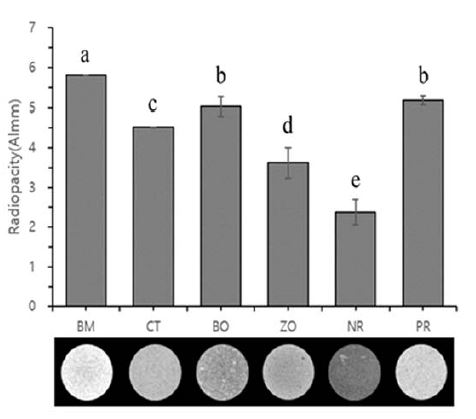
Radiopacity of specimens in comparison with thickness of the aluminum step wedge in mm (Almm). White MTA with four different radiopacifiers; bismuth oxide (BM), calcium tungstate (CT), barium oxide (BO), and zirconium oxide (ZO). The group with no radiopacifier (NR) was used as a negative control and ProRoot MTA (PR) was used as the commercial control. The same lowercase letters indicate no statistical significant differences (p>0.05).
Discussion
Despite its advantages, crown discoloration of MTA in the pulp chamber has been reported in the depth of the material. There have been various reasons for this discoloration, including blood contamination and contact with the solutions routinely used in endodontics (15). Among these various solutions, the hydrogen peroxide used for bleaching and lidocaine used as an anesthetic did not affect the tooth color (16). On the other hand, the hypochlorite solution used for canal irrigation produced a color change when contacting bismuth-containing MTA. When it contacted sodium hypochlorite, it lost oxygen, which led to a dark brown color. It seems that this dark color change could indicate a change from oxide to bismuth metal (6).
The radiopacity of an endodontic material is also one of the most important characteristics for the observation of the radiographic interface between the material and tooth substrate, and it is tested based on the international standard in comparison to an aluminum wedge according to standard. It is quantitatively assayed by comparing a graduated aluminum step wedge with equivalent thickness to determine the optical radiographic density under X-ray exposure (14).
To overcome the discoloration, we replaced the bismuth oxide with other radiopacifiers and investigated the color change and radiopacity after immersion in a hypochlorite solution. Among the radiopacifiers, radio-contrast agents used to enhance the visibility of internal structures under X-ray exposure have been reported to produce allergic reactions. Thus, we selected FDA-approved radiopaque formulations, including barium oxide, bismuth oxide, calcium tungstate, and zirconium oxide (17, 18). The selection of an appropriate radiopacifier in the proper amount requires a thorough understanding of its characteristics and how various radiopaque compounds could affect MTA.
Barium compounds such as barium sulfate have been used as X-ray radiocontrast agents. However, barium oxide reacts violently with water, which makes it unsuitable for water-based cement. The barium compound had a lower density than bismuth oxide (5.72 g/cm3), which showed a lower radiopacity for the same volume and it is an insoluble additive for oil well drilling fluids. In addition, in a purer form, it is used as an X-ray radiocontrast agent for imaging the human gastrointestinal tract. However, soluble barium ions and soluble compounds are poisonous, and water-soluble barium compounds are poisonous, with low doses of barium ions acting as a muscle stimulant and affecting the nervous system (18).
Bismuth is used in various medical devices, although it is more expensive than a barium-based material, it is twice as dense (8.90 g/cm3). Because of its density, a 40% bismuth compound contains only approximately half the volume ratio of a 40% barium sulfate or barium oxide compound. Because bismuth produces a brighter, sharper, higher-contrast image on an X-ray film or fluoroscope than barium, it is commonly used whenever a high level of radiopacity is required (17).
Tungsten has a higher specific gravity than bismuth, and a loading of 60% tungsten has approximately the same volume ratio as a 40% bismuth compound. Because of its density, a device with calcium tungstate can be made highly radiopaque with low loading. White calcium tungstate (density: 6.06 g/mL) showed the potential to replace bismuth oxide, however, it showed a lower radiopacity than bismuth oxide in same percentage. Zirconium oxide (density: 6.52 g/cm3) showed a radiopacity result similar to that of calcium tungstate, with no discoloration.
In terms of discoloration, calcium tungstate and zirconium oxide showed no color change. However, the barium oxide group showed change in color to light gray. The bismuth oxide containing groups (BM and PR) showed the highest color change, turning dark brown. As previously mentioned, the bismuth oxide showed a change by oxidation.
From the results, bismuth oxide showed the highest radiopacity result and the calcium tungstate and zirconium oxide also showed distinguishable results. All the test materials showed radiopacity except the negative control group.
Various alternative radiopaque contrast agents were identified in this study, and the potentials of some materials were confirmed. The further tests are required for confirmation of optimal radiopacifier in terms of both radiopacity and discolorations. Still, it was evident from this study that the calcium tungstate followed by the zirconium oxide could be a choice of radiopacifier.
Conclusion
Within the limits of the present study, the following conclusions can be drawn
1. Color changes of White MTA containing bismuth oxide was significantly greater than White MTA containing other radiopacifier.
2. Color changes of White MTA containing calcium tungstate and barium oxide was significantly lower than White MTA containing bismuth oxide.
3. White MTA containing calcium tungstate and barium oxide showed reduced but adequate level of radiopacity than MTA containing bismuth oxide.
Acknowledgments
This study was supported by a new faculty research seed money grant of Yonsei University College of Dentistry (2019-32-0017).
References
-
Kim YS, Choi SH, Youn KE, Jang JH, Chang HS, Hwang YC, Hwang IN, Oh WM, Lee BN. Effects of various root canal sealers on tooth discoloration and internal bleaching. Korea J Dent Mater. 2019;46(1):1-10.
[https://doi.org/10.14815/kjdm.2019.46.1.1]

-
Lee HI, Choi SH, Jang JH, Chang HS, Hwang YC, Hwang IN, Lee BN, Oh WM. Effects of RetroMTA on osteoblastic differentiation in MC3T3-E1 cells. Korea J Dent Mater. 2018;45(2):97-109.
[https://doi.org/10.14815/kjdm.2018.45.2.97]

-
Kwon YD, Seok SH, Lee SH, Lim BS. Comparison of intraosseous implantation between paste type mineral trioxide aggregates (MTA) and powder-liquid mix type MTA. Korea J Dent Mater. 2017;44(3): 229-46.
[https://doi.org/10.14815/kjdm.2017.44.3.229]

-
Cho YB, A review of the physical, chemical properties of MTA. Kor J Dent Mater. 2015;42(1):51-6
[https://doi.org/10.14815/kjdm.2015.42.1.51]

-
Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25(3):197-205.
[https://doi.org/10.1016/S0099-2399(99)80142-3]

-
Camilleri J. Color stability of white mineral trioxide aggregate in contact with hypochlorite solution. J Endod. 2014;40(3):436-40.
[https://doi.org/10.1016/j.joen.2013.09.040]

-
Felman D, Parashos P. Coronal tooth discoloration and white mineral trioxide aggregate. J Endod. 2013;39(4):484-7.
[https://doi.org/10.1016/j.joen.2012.11.053]

-
Belobrov I, Parashos P. Treatment of tooth discoloration after the use of white mineral trioxide Aggregate. J Endod 2011;37(7):1017-20.
[https://doi.org/10.1016/j.joen.2011.04.003]

-
Guimaraes BM, Tartari T, Marciano MA, Vivan RR, Mondeli RF, Camilleri J, Duarte MA. Color stability, radiopacity, and chemical characteristics of white mineral trioxide aggregate associated with 2 different vehicles in contact with blood. J Endod. 2015;41(6):947-52.
[https://doi.org/10.1016/j.joen.2015.02.008]

-
Valles M, Mercade M, Duran-Sindreu F, Bourdelande JL, Roig M. Influence of light and oxygen on the color stability of five calcium silicate-based materials. J Endod. 2013;39(4):525-8.
[https://doi.org/10.1016/j.joen.2012.12.021]

-
Kang SH, Shin YS, Lee HS, Kim SO, Shin Y, Jung IY, Song JS. Color changes of teeth after treatment with various mineral trioxide aggregate-based materials: an ex vivo study. J Endod. 2015;41(5):737-41.
[https://doi.org/10.1016/j.joen.2015.01.019]

-
Berger T, Baratz AJ, Gutmann JL. In vitro investigations into the etiology of mineral trioxide tooth staining. J Conserv Dent. 2014;17(6):52 6-30.
[https://doi.org/10.4103/0972-0707.144584]

-
Marciano MA, Duarte MA, Camilleri J. Dental discoloration caused by bismuth oxide in MTA in the presence of sodium hypochlorite. Clin Oral Invest. 2015;19(9):2201-9.
[https://doi.org/10.1007/s00784-015-1466-8]

- International Standard ISO, ISO 6876 Dentistry - Root canal sealing materials, Geneva, 2012.
-
Lenherr P, Allgayer N, Weiger R, Filippi A, Attin T, Krastl G. Tooth discoloration induced by endodontic materials: a laboratory study. Int Endod J. 2012;45(10):942-9.
[https://doi.org/10.1111/j.1365-2591.2012.02053.x]

-
Tsujimoto M, Ookubo A, Wada Y, Matsunaga T, Tsujimoto Y, Hayashi Y. Surface changes of mineral trioxide aggregate after the application of bleaching agents: electron microscopy and an energy-dispersive X-ray microanalysis. J Endod. 2011;37(2):231-4.
[https://doi.org/10.1016/j.joen.2010.11.013]

-
Carrodeguas RG, Lasa BV, Del Barrio JS. Injectable acrylic bone cements for vertebroplasty with improved properties. J Biomed Mater Res B Appl Biomater. 2004;68(1):94-104.
[https://doi.org/10.1002/jbm.b.20007]

-
Vazquez B, Ginebra MP, Gil FJ, Planell JA, Lopez Bravo A, San Roman J. Radiopaque acrylic cements prepared with a new acrylic derivative of iodo-quinoline. Biomaterials 1999;20(21):2047-53.
[https://doi.org/10.1016/S0142-9612(99)00108-8]