Effect of various surface treatments by the sol-gel method on the bond and flexural strengths of zirconia-porcelain
Abstract
This study investigates the effect of a silica coating using the sol-gel method on the bonding between zirconia and porcelain. The specimens were manufactured by precise machining in accordance with the international standard (ISO 9693:2019) using commercially available zirconia (IPS-E-max-ZirCAD). The specimens were then classified into an untreated group (control), and 50 µm sandblasted, 110 µm sandblasted, 0.5 mol% sol-gel, and 0.25 mol% sol-gel treated groups. The control group was subjected to grinding treatment only, the sandblasted group used aluminum oxide, and the sol-gel group was impregnated with the test solution, dried, and sintered according to the manufacturer’s instructions. The sintered specimen underwent a grinding process, and after being layered on the zirconia specimen using a dedicated mold, it was fired according to the manufacturer's instructions. In this study, the strength was measured using a universal testing machine by applying a load until fracture occurred at the center of the specimen. Comparison of the results for the poor bonding between the initial zirconia and porcelain revealed that the sol-gel groups showed the highest bonding strength. In the absence of silica treatment, the control and 110 µm sandblasted groups showed a significantly lower bonding strength that that of the sol-gel treated groups. The 50 µm sandblasted group showed a slightly higher bonding strength than the control and 110 µm sandblasted groups owing to the rough surface of the 50 µm sandblasted specimens. When a load was continuously applied and the zirconia was finally destroyed, the sol-gel group showed lower flexural strengths (different from the initial strength) owing to the change in the elastic modulus due to silica penetration. The flexural strengths exhibited by the sol-gel treated groups were statistically significantly lower than those of the control and 110 µm sandblasted groups. The remaining three groups showed flexural strengths that did not significantly exceed the manufacturer’s strength value. Thus, when zirconia and porcelain were combined and silica was penetrated by the sol-gel method, the bonding strength increased, but the final flexural strength of zirconia was lowered due to the change in the elastic modulus.
초록
본 연구는 졸-겔법에 의한 실리카 코팅이 지르코니아와 포세린 간의 결합에 미치는 영향에 대해 조사하고자 하였다. 현재 상용화 되어 있는 지르코니아(IPS, E-Max, ZirCAD)를 사용하여 국제 표준(ISO 9693:2019) 규격에 맞추어 정밀한 기계가공을 통하여 시편을 제작하였다. 시편은 미처리 그룹(대조군), 50 µm 샌드블라스터, 110 µm 샌드블라스터, 0.5 mol% 졸-겔, 및 0.25 mol% 졸-겔 처리한 그룹으로 분류하였다. 대조군은 연마 처리만 하였으며, 샌드블라스터 그룹은 산화 알류미늄을 사용하고, 졸-겔 그룹은 시험수용액에 함침과 건조과정 이 후 제조사의 지시대로 소결 과정을 거쳤다. 소결된 시편은 연마 과정을 거치고, 전용 몰드를 사용하여 지르코니아 시편 위에 축성 후 제조사의 지시대로 소성하였다. 본 연구에서는 만능시험기를 사용하여 시편 중앙 부위에서 파절이 일어날 때까지 하중을 가하여 강도를 측정하였다. 초기 지르코니아와 포세린과의 결합이 떨어지는 결과를 비교해보면, 0.5 mol% 졸-겔 그룹과 0.25 mol% 졸-겔 그룹은 다른 나머지 세 그룹에 비해 높은 결합강도 값을 나타냈으며, 특히 대조군 및 110 µm 샌드블라스터 그룹과 통계적 유의차를 보여주었다. 대조군 및 샌드블라스터 그룹들은 실리카를 처리하지 않아 상대적으로 낮은 결합강도 값을 보여주었지만, 이 중 50 µm 샌드블라스터 그룹은 거친 표면으로 인해 약간 높은 결과 값을 나타냈다. 이 후 꾸준히 하중을 가하여 최종적으로 지르코니아가 파괴되었을 때의 결과는 결합강도와는 다르게 실리카 침투로 인한 탄성계수의 변화로 인해 졸-겔 그룹들이 낮은 파절강도 값을 보여주었다. 이 또한 졸-겔 그룹들은 대조군 및 110 µm 샌드블라스터 그룹과 통계적 유의차를 보여주었다. 나머지 세 그룹들은 제조사에서 발표된 파절강도 값과 크게 상회하지 않은 값을 나타냈다. 이 결과로써 지르코니아와 포세린 결합시 졸-겔법에 의핸 실리카 침투를 한 경우 결합강도 값은 높아지나 탄성계수 변화에 의해 지르코니아 최종 파절강도는 낮아지는 것으로 결론지었다.
Keywords:
Sol-gel, Zirconia, Porcelain, Bond strength, Flexural strength키워드:
졸-겔, 지르코니아, 포세린, 결합강도, 파절강도Introduction
Zirconia is white and opaque like chalk at room temperature and exists in the monoclinic, tetragonal, and cubic phases as the temperature increases. Additionally, zirconia offers abrasion resistance, biocompatibility, chemical stability, and has a high melting point; therefore it is widely used as a heat-resistant material, and is known to have very high fracture strength (1). Polycrystalline zirconia stabilized by adding 3-5 mol% yttria (yttrium tetragonal polycrystals, Y-TZP) is widely used in dentistry.
In the fabrication of restorations using zirconia, owing to the low aesthetics of zirconia, it is manufactured as an internal structure and is widely applied in the form of a structure similar to that of a porcelain-fused metal (PFM) in which porcelain is applied externally. The PFM is also aesthetically supplemented by zirconia, but due to the low tensile strength of the ceramic, the bonding strength decreases and frequent fracture occurs (2, 3). It has also been reported that it is susceptible to fracture due to the drop-off or stress concentration at the interface of zirconia and the external porcelain located inside (4). These issues are exacerbated by the difference in thickness between zirconia and porcelain, the two ceramic layers, heat shrinkage and expansion due to sintering and cooling, and the residual stress caused by unsuitable shape setting in computer-aided design (CAD) using professional dental software (5-7). Although zirconia subjected to primary sintering is easy to cut, shrinkage (18-20%) occurs after complete sintering. To compensate for this shrinkage, a larger design is prepared in advance during machine milling, following which the manufacturing process is carried out. Thus, the precision may reduce and the thin part can easily break.
To compensate for these disadvantages, bonding has been induced by physically forming mechanical irregularities before sintering. In addition, a technology that induces bonding with porcelain by forming an intermediate layer after applying the lining material recommended by the manufacturer to the core and firing at a high temperature has been applied. It has been reported that zirconia and porcelain form ionic bonds, and as the temperature and time increase during sintering, silicon ions diffuse into the lattice structure of zirconia, which may increase its bonding strength (8).
The color and transparency of dentin can be obtained by penetrating the opaque zirconia core with an additive to complement the color. Recently, by adding a large amount of yttria to each layer, the color and transparency could be reproduced when manufacturing multiple blocks, thereby further improving the aesthetics of the prosthesis. However, due to the high opacity of zirconia itself, a single zirconia structure is still used, all zirconia is commercialized, and aesthetic limitations still exist. In addition, the wear of the antagonist, the problem of color tone, and the absence of structural standards for clinical stability are among the reasons that render the use of the multiblock type challenging. To overcome these problems, complete a precise prosthetic restoration, and perfectly reproduce the transparency and aesthetics, porcelain veneering is ultimately required.
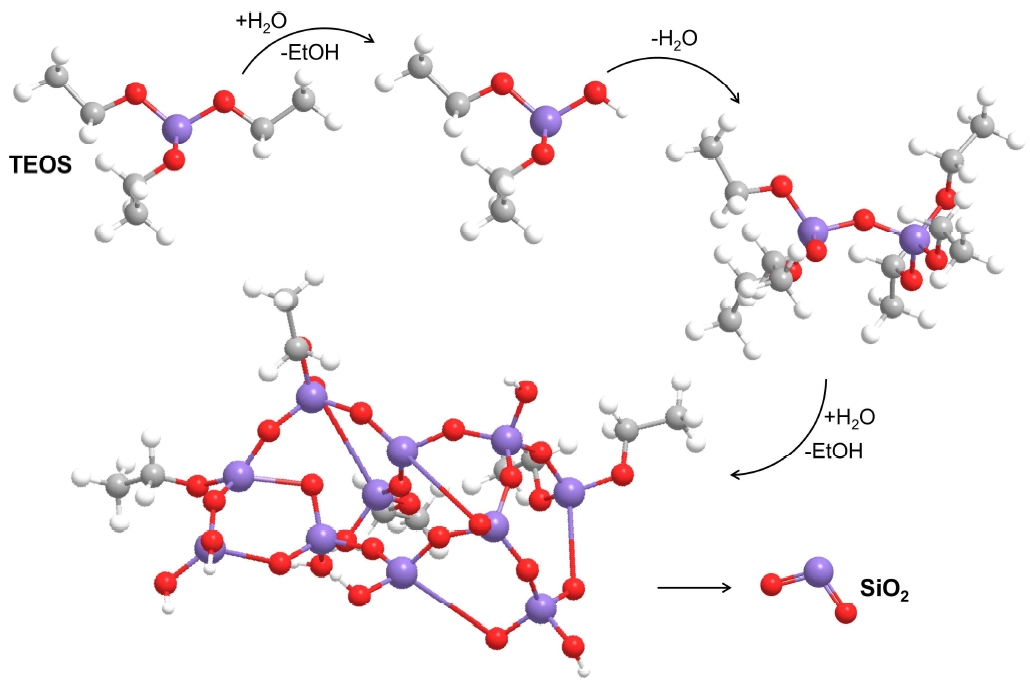
There are extensive past and ongoing studies on improving adhesion to porcelain through various treatments on the zirconia surface (9). Zirconia and porcelain either bond mechanically or through a method that relies on the corrosion and frictional forces of the adhesive. However, since zirconia does not contain silica, the difference in results will depend on the conditions and research environment is very relatively (10). The sol-gel technique using a non-aqueous medium, which is known to not only increase the structural homogeneity of zirconia, but also increase the load-bearing capacity of the restoration, was applied in this study (11-13). In general, the sol-gel process is a method of producing a solid phase from small molecules, and it was carried out on the assumption that the bonding strength could be increased by converting the sol-state zero-gel film into a dense film form by heat treatment. In this study, considering the possibility of fracture due to the brittleness between zirconia and porcelain, the relationship between the bonding and fracture strengths was investigated by applying various surface treatments to zirconia and porcelain, which are widely used clinically, by the sol-gel method with a polymerization of an alkoxide (Figure 1).
Materials and Methods
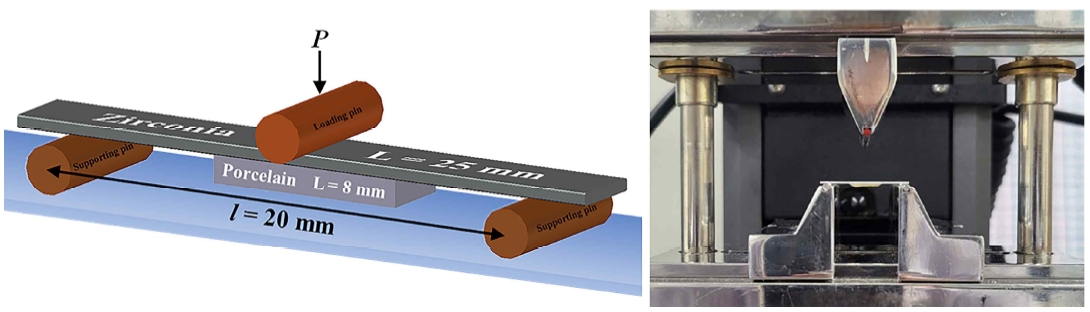
Specimens were prepared through precise machining using a dedicated milling machine (K5, vhf, Germany) to match the zirconia block (IPS, E-Max and Zir-CAD, Ivoclar Vivadent, Schaan, Liechtenstein) to the standard presented in ISO 9693:2019 (Dentistry-Compatibility testing for metal-ceramic and ceramic-ceramic). Zirconia in the chalk form is known to shrink by 18-20% after sintering; therefore the size of the final specimen after processing was set to 25(±1) × 3(±0.1) × 0.5(±0.05) mm3 (Figure 2). The machining parallelism error of the specimen was determined to be 0.02 mm or less.
The 45 specimens thus prepared were randomly divided into five groups (n=9), and divided into an untreated group (CTRL), 50 µm sandblasted (50SB), 110 µm sandblasted (110SB), 0.5 mol% sol-gel (0.5SG), and 0.25 mol% sol-gel (0.25SG) groups (Table 1). Silica solutions for the 0.5SG and 0.25SG groups were prepared by mixing distilled water, ethanol, and hydrochloric acid in certain ratios with different concentrations (0.5 and 0.25 mol) of tetraethyl orthosilicate (TEOS, Si(OC2H5)4) (Table 2). The CTRL group was polished, and the 50SB and 110SB groups were sprayed with aluminum oxide for 2 s at a distance of 10 mm at a pressure of 2 bar. The 0.5SG and 0.25SG groups were immersed in the prepared dedicated test solution for 30 min, dried at 60 ℃ for approximately 40 min, and sintered according to the manufacturer's instructions (Table 3).
The sintered specimens were polished using 600-grit and 800-grit SiC abrasive papers on a polishing machine (Ecomet3, Buehler, Lake Bluff, IL), and 45°chamfering was performed using 800-grit abrasive paper to prevent a decrease in strength due to the edge of the specimen.
For porcelain veneering, a sintering process was performed in accordance with the process conditions laid down by the manufacturer after layering the upper part of the zirconia specimen using a mold considering shrinkage (Table 4). A porcelain specimen of dimensions 8(±0.1) × 3(±0.1) × 1.1(±0.1) mm3 was fabricated according to the standard (Figure 1) and dried at room temperature to prevent low-temperature degradation, then washed under ultrasonic waves, and stored for 2 days in distilled water at 37 ℃. Finally, this specimen was subjected to a three-point bending test (Model 5942; Instron®, Norwood, MA) (Figure 2).
All data were showed as means ± standard deviation and statistically analyzed using IBM SPSS Statistics (Version 26. IBM Corp., Armonk, NY). One-way ANOVA followed by Tukey post-hoc test was used and the significant value (p-value) was set as p<0.05.
Results and Discussion
Dental zirconia exhibits a high strength and toughness similar to metals; therefore, it is widely used as a core material to replace the PFM. Recently, owing to the development of materials, multi-zirconia with increased yttria content (4–5 mol%) has been developed, which has improved aesthetics compared to general zirconia (3 mol%) but has a relatively low strength. Because of the opaque nature of early zirconia, ceramic layering is essential to provide transparency to the anterior region, which requires aesthetics (14). Additionally, because it is vulnerable to tensile forces, an appropriate thickness is essential for the core, and many studies are being conducted to improve the bonding force between the two ceramic layers. Therefore, before porcelain veneering, physical or chemical surface treatment methods, such as using a lining material recommended by each manufacturer on the surface, or various methods to induce roughness or corrosion, are being applied and studied. However, fractures between zirconia and porcelain are frequently reported, and further research is needed to mitigate these (15). In this study, considering the possibility of fracture due to brittleness, the sol-gel method was performed on the core surface of zirconia-porcelain, which is widely used clinically, to improve the bonding strength between the two ceramics, and the effect of the silica coating on the bonding strength was studied.
The maximum peak strength measurements that confirm the bonding force are shown in Figure 3. After impregnating the zirconia specimen with silica, the SG groups show the highest bonding strength (mean value) with porcelain. This treatment appears to have increased the chemical bonding with porcelain by infiltrating silica-like threads between the particles in the choke state of zirconia. In particular, the SG groups showed a statistically significant difference from the CTRL and 110SB groups. Silica-free zirconia specimens, such as those in the CTRL, 50SB, and 110SB groups, are known to present difficulties with inducing chemical bonding. However, when silica is generated in the choke state, its strength is increased by combining the components of porcelain and silica. Moreover, after sandblasting, the 50SB group (295.1±71.1 MPa) shows a slightly higher bonding strength than the 110SB group (246.4±59.1 MPa) to which porcelain was applied. The bond here is considered to be a fine mechanical bond because the alumina particles are larger; hence fine particles are embedded on the surface or a rough surface is formed.
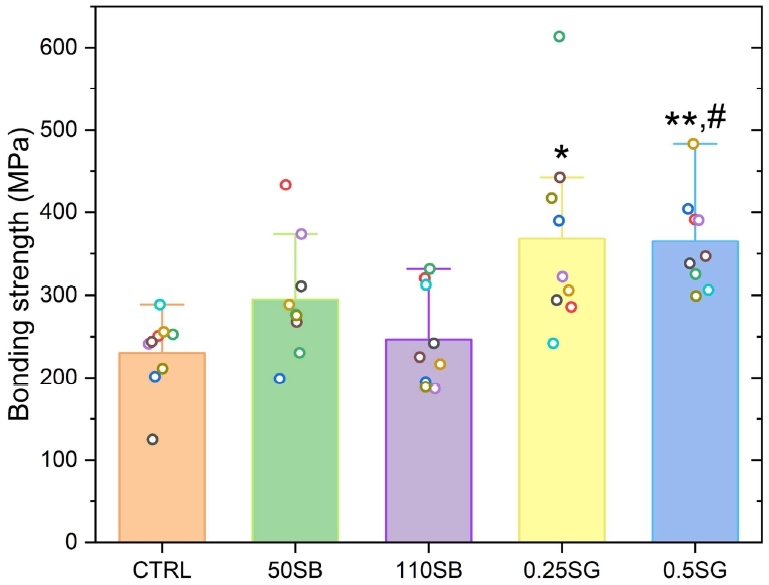
Bonding strengths of zirconia-porcelain. CTRL group vs. 0.5SG group, #p<0.05. 110SB group vs. 0.25SG and 0.5SG groups, *p<0.05, **p<0.001
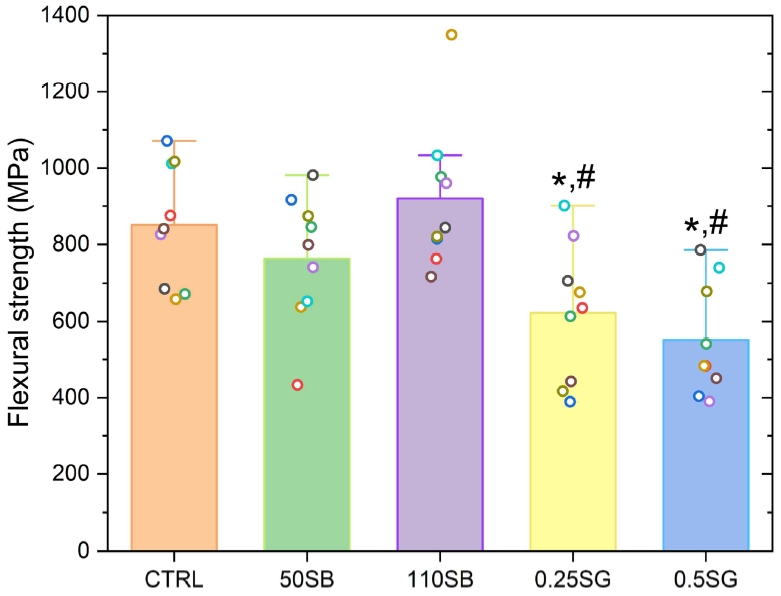
Figure 4 shows the flexural strength at which zirconia finally fractures. The group with the lowest strength is the 0.5SG group (550.7±147.6 MPa), which is possibly attributed to the change in the elastic modulus of the existing zirconia particles as silica infiltrates between the original zirconia particles during sintering. In addition, the SG groups showed statistically significantly lower flexural strength than that of the CTRL and 110 µm sandblasted groups. According to the structure obtained after the three-point bending test, cracking starts at the lower part where the tensile stress is concentrated, but in zirconia with a high fracture strength, it is judged that the fused ceramic part falls first and fractures from the moment the force is applied. As the content of silica increases, the resistance to fracture increases.
Conclusions
- 1. From the results at the first peak immediately before the porcelain fracture was calculated, the CTRL and 110SB groups showed the lowest bending strength, and the 50SB group showed a slightly higher bending strength than the two preceding groups.
- 2. The 0.25SG and 0.5SG groups showed significantly higher bending strengths than the other groups.
- 3. The strengths of the CTRL, 50SB, and 110SB groups exceeded the strength values declared by the manufacturer, whereas the strengths of the 0.25SG and 0.5SG groups were lower.
The bending strengths of the silica-impregnated zirconia specimens were higher than those of the CTRL, 50SB, and 110SB groups, and there was no significant difference in the results among the CTRL, 50SB, and 110SB groups. Thus sol-gel coating with an appropriate concentration of silica solution improved the bonding strength between the zirconia and porcelain layers. However, the final bending strength of zirconia was affected by the reaction of the silica layer produced by the sol-gel coating during sintering with zirconia, and by weakening due to cohesive failure when the porcelain layer was separated.
Acknowledgments
This paper was supported by research funds for newly appointed professors of Gangneung-Wonju National University in 2021; National Research Foundation of Korea (NRF) grants funded by the Ministry of Science and ICT under contract NRF-2018M3C1B7021994 (Bioinspired Innovation Technology Development Project) and the Ministry of Education contract NRF-2020R1I1A1A01070982 (Basic Science Research Program).
References
-
Balakrishnan A, Panigrahi BB, Sanosh KP, Chu M-C, Kim TN, Cho S-J. Effect of high thermal expansion glass infiltration on mechanical properties of alumina-zirconia composite. Bull Mat Sci. 2009;32(4):393.
[https://doi.org/10.1007/s12034-009-0057-1]

-
Kelly JR, Tesk JA, Sorensen JA. Failure of All-ceramic Fixed Partial Dentures in vitro and in vivo: Analysis and Modeling. J Dent Res. 1995;74(6):1253-8.
[https://doi.org/10.1177/00220345950740060301]

- Larsson C, Vult von Steyern P Fau - Sunzel B, Sunzel B Fau - Nilner K, Nilner K. All-ceramic two- to five-unit implant-supported reconstructions. A randomized, prospective clinical trial. Swed Dent J. [0347-9994 (Print)].
-
Campos F, Souza RO, Bottino MA, Ozcan M. Fracture Strength, Failure Types, and Weibull Characteristics of Three-Unit Zirconia Fixed Dental Prostheses After Cyclic Loading: Effects of Veneering and Air-Abrasion Protocols. Int J Periodontics Restor Dent. 2016;36(6):901-8.
[https://doi.org/10.11607/prd.2524]

-
Fukushima KA, Sadoun MJ, Cesar PF, Mainjot AK. Residual stress profiles in veneering ceramic on Y-TZP, alumina and ZTA frameworks: measurement by hole-drilling. Dent Mater. 2014;30(2):105-11.
[https://doi.org/10.1016/j.dental.2013.10.005]

-
Durand J-C, Jacquot B, Salehi H, Margerit J, Cuisinier FJG. Confocal Raman microscopy and SEM/EDS investigations of the interface between the zirconia core and veneering ceramic: the influence of a liner and regeneration firing. J Mater Sci-Mater Med. 2012;23(6):1343-53.
[https://doi.org/10.1007/s10856-012-4616-4]

-
Mainjot AK, Schajer GS, Vanheusden AJ, Sadoun MJ. Influence of cooling rate on residual stress profile in veneering ceramic: measurement by hole-drilling. Dent Mater. 2011;27(9):906-14.
[https://doi.org/10.1016/j.dental.2011.05.005]

-
Tholey MJ, Swain MV, Thiel N. SEM observations of porcelain Y-TZP interface. Dent Mater. 2009;25(7):857-62.
[https://doi.org/10.1016/j.dental.2009.01.006]

-
Farhan FA, Sulaiman E, Kutty MG. Effect of new zirconia surface coatings on the surface properties and bonding strength of veneering zirconia substrate. Surf Coat Technol. 2018;333:247-58.
[https://doi.org/10.1016/j.surfcoat.2017.10.030]

-
Juntavee N, Dangsuwan C. Role of coefficient of thermal expansion on bond strength of ceramic veneered yttrium-stabilized zirconia. J Clin Exp Dent. 2018;10(3):e279-e86.
[https://doi.org/10.4317/jced.54605]

-
Campos TM, Ramos NC, Machado JP, Bottino MA, Souza RO, Melo RM. A new silica-infiltrated Y-TZP obtained by the sol-gel method. J Dent. 2016;48:55-61.
[https://doi.org/10.1016/j.jdent.2016.03.004]

-
Zhang Y, Kim JW. Graded zirconia glass for resistance to veneer fracture. J Dent Res. 2010;89(10):1057-62.
[https://doi.org/10.1177/0022034510375289]

-
Liu D, Pow EHN, Tsoi JK, Matinlinna JP. Evaluation of four surface coating treatments for resin to zirconia bonding. J Mech Behav Biomed Mater. 2014;32:300-9.
[https://doi.org/10.1016/j.jmbbm.2013.12.011]

-
Von Steyern PV, Ebbesson S, Holmgren J, Haag P, Nilner K. Fracture strength of two oxide ceramic crown systems after cyclic pre-loading and thermocycling. J Oral Rehabil. 2006;33(9):682-9.
[https://doi.org/10.1111/j.1365-2842.2005.01604.x]

-
Sim JH, JB Lee, Hwang SS. Effect of glazing on the flexural strength of lithium disilicate glass ceramics. Korean J Dent Mater. 2019;46(4):185-93.
[https://doi.org/10.14815/kjdm.2019.46.4.185]