The stimulatory effect of Angelica tenuissima Nakai in osteoblastic/odontoblastic differentiation of human periodontal ligament stem cells
Abstract
Angelica tenuissima Nakai (ATN) is an herbal medicine used to treat toothache, headaches, and cold symptoms. However, the therapeutic effects of ATN have not been thoroughly identified. The purpose of this research was to explore the effect of ATN in osteo/odontoblastic differentiation of human periodontal ligament stem cells (hPDLSCs). The influences of ATN on the differentiation and proliferation of hPDLSCs were evaluated using alizarin red S staining, real-time PCR, western blot and MTT assay. ATN promoted osteo/odontoblastic differentiation of hPDLSCs, accelerated mineral nodule formation in vitro, and displayed no toxicity at higher concentrations. The mRNA expression levels of alkaline phosphatase (ALP), type I collagen (COL1), osteopontin (OPN), dentin matrix acidic phosphoprotein 1 (DMP-1), and dentin sialophosphoprotein (DSPP) in hPDLSCs and the protein levels of osteocalcin (OCN), DSPP, DMP-1, and Runx2 were significantly higher after ATN treatment. HEK293 cells overexpressing the osterix (OSX) gene and treated with ATN (100 μg/mL) showed an increase in BSP promoter activity. Those results suggest that ATN enhances the osteo/odontoblastic differentiation of hPDLSCs. These therapeutic properties of ATN can serve as a theoretical basis for further research on the applicability of ATN in periodontal tissue regeneration.
초록
Angelica tenuissima Nakai (ATN)는 치통, 두통, 감기 증상을 치료하는 데 사용되는 천연물로 약리학적 기전 및 질환에 대한 다양한 치료효과가 완전히 규명되지 않았다. 본 연구의 목적은 ATN이 사람 치주인대줄기세포의 조골/상아질모세포 분화에 미치는 영향을 탐색하는 것이었다. 치주인대줄기세포의 분화 및 증식에 대한 ATN의 영향은 Alzarin red S 염색, 실시간 PCR, 웨스턴 블랏 및 MTT 분석을 사용하여 평가되었다. ATN은 치주인대줄기세포의 조골/상아질모세포 분화를 촉진하고, 미네랄 결절 형성을 가속화하였으며, 고농도에서 독성을 보이지 않았다. 또한 ATN은 치주인대줄기세포에서 알칼리 포스파타아제(ALP), 콜라겐 타입 I(COL1), 오스테오폰틴(OPN), 덴틴 매트릭스 산성 인단백질 1(DMP-1), 덴틴시알로포스포단백질(DSPP)의 mRNA 발현 및 오스테오칼신(OCN), DSPP, DMP-1, Runx2의 단백질 수준을 유의하게 증가시켰다. 추가로, osterix유전자를 과발현하고 ATN (100 µg/mL)으로 처리한 HEK293 세포는 BSP 프로모터 활성이 증가하는 것을 보였다. 이러한 결과는 ATN이 치주인대줄기세포의 조골/상아질모세포 분화를 향상시킨다는 것을 의미한다. ATN의 이러한 치료적 특성은 치주 조직 재생에서 ATN의 적용 가능성에 대한 추가 연구를 위한 이론적 근거로 작용할 수 있다.
Keywords:
Angelica tenuissima Nakai, human periodontal ligament stem cells, osteoblastic/odontoblastic differentiation키워드:
치주인대줄기세포, 조골/상아질모세포 골분화Introduction
Angelica tenuissima Nakai (ATN), belonging to the Apiaceae family, is a traditional medicine used to treat headaches, toothaches, and diarrhea in East Asian (1, 2). The hepatoprotective and antioxidative activities of ATN have been well studied (3, 4), and its anti-inflammatory role as a cyclooxygenase-2 inhibitor has also been reported (1). The anti-inflammatory properties of ATN are attributed to its components, including ferulic acid and various essential oil compounds, such as limonene, 3-butylidenephthalide, γ-terpinene, ligustilide, senkyunolide, and neocnidilide (5, 6). Ferulic acid functions to neutralize lipopolysaccharide-induced inflammatory response (7), whereas ligustilide prevents osteoarthritis by suppressing NF-κB activation via the PI3K/AKT pathway (8).
Pulpal tissue inflammation associated with periodontal disease is often the root cause for toothaches (9). Dental caries lead to acute pulpitis, inflammation of the dental pulp and causes critical pulp pain (10). Damaged teeth restore function and appearance relatively well after endodontic and restorative treatment (11). Periodontitis is a common chronic inflammatory disease that results in the degeneration of periodontal tissue (12). Periodontitis treatment involves preventing infection as well as the regeneration of damaged periodontal tissue, including the periodontal ligament, cementum, and alveolar bone. General treatments for periodontal disease include basic treatment, guided bone regeneration (GBR), and guided tissue regeneration (GTR) (13). However, these methods have poor clinical predictability and the outcomes are limited. Interestingly, stem cell therapy has shown improved clinical outcomes for periodontitis (14, 15).
Among the most reliable sources of mesenchymal stem cells (MSCs) in the human body, human periodontal ligament stem cells (hPDLSCs) have the ability to differentiate into periodontal ligament (PDL), bone, and cementum-forming lineages (16). These cells display self-renewal and multipotential abilities that are useful in periodontal tissue treatment therapies and regenerative medicine (17, 18). The destruction of periodontal tissue in periodontitis is a consequence of inflammatory reactions stemming from prevalent bacterial infections (19). Thus, ATN and its anti-inflammatory properties, which has been shown to be based on cyclooxygenase-2 suppression, may has significant roles in periodontitis treatment (1). However, to the present date, no studies have elucidated the roles of ATN on periodontal tissue regeneration. Our study objects to examine the influences of ATN on periodontal tissue regeneration and to understand its potential in the management of periodontal disease.
Materials and Methods
1. Preparation of ATN extract
ATN was obtained from Kwang Myung Dang (Ulsan, Korea) and was verified for its authenticity in the Department of Herbology, College of Korean Medicine, Wonkwang University. After adding 1L of distilled water to 75 g of ATN, the mixture was decoated at 100 ℃ for 2 h, concentrated and freeze-dried to obtain ATN extract. The dried extract production was 13.7 g.
2. Preparation of hPDLSCs
The experiment was conducted using teeth obtained from healthy young males over the age of 20. The research protocol was approved by the Institutional Review Board (IRB No. WKDIRB-201708-02) of Wonkwang University in Iksan, South Korea. hPDLSCs were isolated from the root surface using a scalpel and the isolated tissue was digested in alpha-modified Eagle’s medium (α-MEM; Gibco BRL, Grand Island, NY, USA) containing 4 mg/mL dispase (Boehringer, Mannheim, Germany) and 3 mg/mL collagenase type I (Worthington Biochem, Freehold, NJ, USA), for 1 h at 37 ℃. The cell suspension was passed through a 70 μm strainer (Falcon BD Labware, Franklin Lakes, NJ, USA) to form a single-cell suspension. Cells were cultured in a 100 mm dish with α-MEM contained 10% fetal bovine serum (Gibco BRL), 100 μM L-ascorbic acid 2-phosphate (Sigma-Aldrich, St. Louis, MO, USA), 2 mM L-glutamine (Gibco BRL), 100 U/ml penicillin, and 100 μg/mL streptomycin (Biofluids, Rockville, MD, USA), at 37 ℃ in 5% CO2. The fourth or fifth passage of the cells was used for experiments.
3. Flow cytometric analysis
The immunophenotype of the hPDLSCs was characterized by flow cytometry. The expression of surface markers related to mesenchymal stem cells in passage 3 was analyzed. Cells in the fourth passage (1.0×106 cells) were blocked for 30 min in phosphate-buffered saline (PBS) containing 2% fetal bovine serum (Gibco BRL). The cells were then cultured at 4 ℃ for 2 hours with antibodies specific to CD34, CD13, CD90 or CD146. All antibodies were obtained from BD Biosciences (San Jose, CA, USA). Flow cytometry was performed using a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). Percentages of CD13+, CD90+, CD146+, and CD34-cells were measured.
4. Osteogenic, chondrogenic, and adipogenic differentiation
To evaluate the ability of osteogenesis, chondrogenesis, and adipogenesis, hPDLSCs were cultured in StemPro Osteogenesis, Chondrogenesis, and Adipogenesis differentiation kit media (Gibco BRL), respectively, with the appropriate supplements. At week 3, the cells were washed with PBS and fixed in 3.7% paraformaldehyde for 10 min. The cells were consequently stained with 2% Alizarin Red S stain (Sigma-Aldrich), 1% Alcian Blue (Sigma-Aldrich), and 0.3% Oil Red O dye (Sigma-Aldrich) to detect osteogenic, chondrogenic, and adipogenic differentiation, respectively. The cells were then visualized with a light microscope.
5. Cytotoxicity and migration assay
The cytotoxicity of ATN was evaluated by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. hPDLSCs were incubated for 24 or 72 h in 96-well plates with various concentrations of ATN. After adding 20 μL of MTT (5 mg/mL) solution to each well of a 96-well plate, the cells were cultured for 2 h. The supernatant was removed, then isopropanol (100 μL) was added. Subsequently, the soluble MTT crystals were quantitatively analyzed by measuring absorbance at 490 nm using a SpectraMax 190 (Molecular Devices, San Jose, CA, USA). To evaluate the influnces of ATN on hPDLSC migration and motility, wound healing assays were accessed on hPDLSC monolayers. The cells were grown to 80% confluence in a 6-well plate. Cell monolayers were wounded using a plastic tip (1 mm). Cell debris was removed by washing twice with PBS and then the monolayer was incubated with ATN (100 μg/mL) or without ATN. Cell migration to the wound surface was evaluated by microscope at 0, 12, 18, and 24 h after the wound.
6. Alizarin red S staining
hPDLSCs were cultured in 24-well plates in α-MEM containing 10% FBS at an initial density of 4×104 cells/well, until reaching 95% confluence. For mineralization, the cells were cultured in osteogenic differentiation medium containing 50 μg/mL ascorbic acid, 10 mM β-glycerophosphate, and 100 nM dexamethasone (Sigma-Aldrich). After 3 weeks, the accumulation of mineral nodules was detected by staining with 2% alizarin red S (Sigma Aldrich) at pH 4.2. For measuring calcium content, the cells were destained by adding 3 mL of 10 mM sodium phosphate solution to each stained well, followed by a 30 min incubation at room temperature. The destained samples were transferred to a 96-well plate and their absorbance was then measured at 405 nm.
7. Western blot analysis
hPDLSCs (1.0×106 cells/dish) were cultured in osteogenic differentiation media with or without ATN (100 μg/mL) for 3 weeks. The concentration of the extracted protein was quantified using DC Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein (30 μg/lane) were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to a polyvinylidene fluoride membrane (GE Healthcare, Buckinghamshire, UK). Primary antibodies against runt-related transcription factor 2 (Runx2), α-tubulin (Cell Signaling Technology, Danvers, MA, USA), bone sialoprotein (BSP), osteopontin (OPN), osteocalcin (OCN), dentin sialophosphoprotein (DSPP), and dentin matrix protein 1 (DMP-1) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used. Blots were developed using horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology) and analyzed by an LAS-4000 (Fujifilm, Tokyo, Japan).
8. RNA preparation and real-time poly chain reaction (PCR) analysis
To evaluate gene expression levels in ATN-treated differentiated hPDLSCs, 1.0×106 cells were seeded in a 60-mm culture dish and cultured for 3 weeks under osteogenic differentiation induction conditions. Total RNA was extracted by the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). cDNA was synthesized using an oligo (dT) primer and reverse transcriptase (Takara, Nojihigashi, Japan). RT-PCR was conducted in a StepOne Real-Time PCR System (Molecular Devices, San Jose, CA, USA) using TOPreal qPCR 2X PreMIX (SYBR Green with low ROX). Samples were incubated at 95 ℃ for 30 s, followed by 40 cycles of 95 ℃ for 5 s and 60 ℃ for 30 s. The relative expression level of each gene was calculated using the 2-ΔΔCt method. The primer sets used in this assay are listed in Table 1.
9. Luciferase reporter assays
A luciferase reporter plasmid, containing a pCMV-β-gal plasmid, the promoter for bone sialoprotein (BSP-Luc), and a combination of osterix (OSX) expression plasmids, were transfected into HEK293 cells. Transfected cells were treated, either with or without ATN (100 μg/mL) for 24 h, and then lysed to assess luciferase activity using a Luciferase Reporter Assay Kit (Promega, Madison, WI). Transfection efficiency was normalized using β-galactosidase.
10. Statistical analysis
Statistics were analyzed using GraphPad Prism 5.03 software (La Jolla, CA, USA). Experimental results are expressed as mean±standard deviation. Statistical significance was set at p<0.05.
Results
1. hPDLSCs have multi-lineage differentiation potential
To access their multi-lineage differentiation capacity, osteogenic, chondrogenic, and adipogenic differentiation were induced with respective differentiation media as previously described (20). After 3 weeks of induction, hPDLSCs differentiated into alizarin red S-positive mineral deposits, alcian blue-positive nodules, and oil red O-positive lipid droplets throughout the adherent layers (Figure 1A). Additionally, flow cytometric analysis revealed high rates of positive expression of the mesenchymal stem cell markers: CD13 (97.79%), CD90 (99.55%), and CD146 (90.85%), whereas CD34, an MSC-negative marker of primitive hematopoietic progenitors and endothelial cells was confirmed as 0.18% expression of hPDLSCs (Figure 1B).
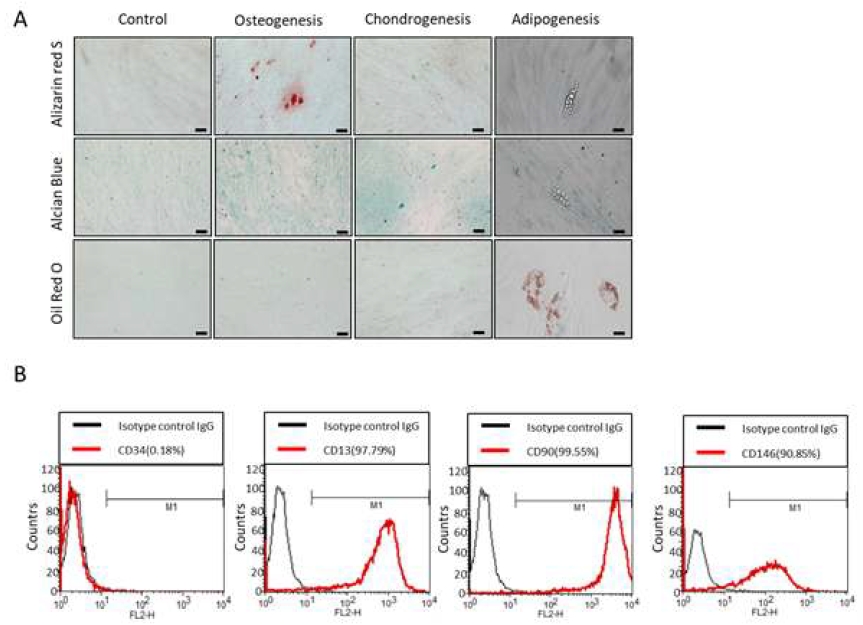
Multi-lineage differentiation potential of human periodontal ligament mesenchymal stem cells (hPDLSCs) (A) hPDLSCs were cultured in osteogenic, chondrogenic, and adipogenic differentiation media for 3 weeks, respectively. To assess the pluripotency of hPDLSCs, Alizarin red S staining, Alcian Blue staining, and Oil red O staining were performed. The scale bar is 100 μm. (B) Flow cytometric analysis of hPDLSCs confirmed positively expressed CD13, CD90, and CD146, while negatively expressed CD34. The percentages of cells to the right of the M1 gate were measured for positive expression (n=3).
2. ATN does not affect the proliferation and migration of hPDLSCs
To investigate the proliferative effect of ATN on hPDLSCs, cells were treated with the various concentrations of ATN. After 24 or 72 h of culture, the MTT cytotoxicity test was performed. We observed that ATN displayed no toxicity at high concentrations (Figure 2A). Furthermore, wound-healing assay was used to analyze the effect of ANT on migration of hPDLSCs. Confluent monolayers of hPDLSCs were cultured with or without ATN for 0, 12, 18, or 24 h, and the migration distances were measured. After 24 h, ATN-treated cells showed no differences from the control group (Figure 2B).
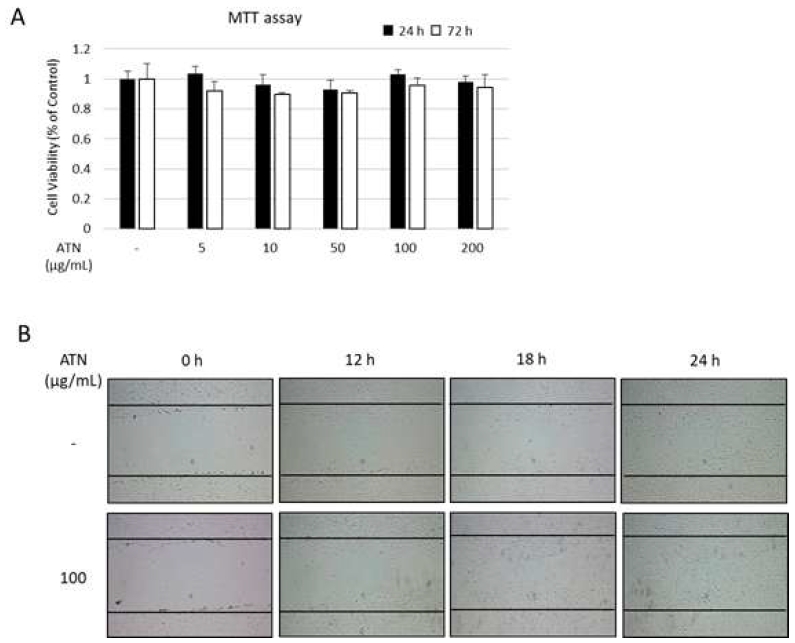
Effect of ATN on the proliferation/migration of human periodontal ligament mesenchymal stem cells (hPDLSCs) (A) To determine the in vitro cytotoxicity of ATN, hPDLSCs were incubated with indicated concentrations of ATN for up to 72 h. (B) Confluent monolayers of hPDLSCs were wounded by scratching and cultured with or without ATN (100 μg/mL) for up to 24 h.
3. ATN increases mineralized nodule formation of hPDLSCs
To explore the stimulatory effect of ATN on osteoblast differentiation, we cultured hPDLSCs in osteogenic differentiation medium with or without ATN (25, 50, or 100 μg/mL). The formation of bone mineral nodules was evident in 100 μg/mL of ATN-treated group compared to the untreated cells (Figure 3A). In addition, the highest calcium content was also observed in the 100 μg/mL ATN-treated group after destaining of Alizarin red S (Figure 3B).
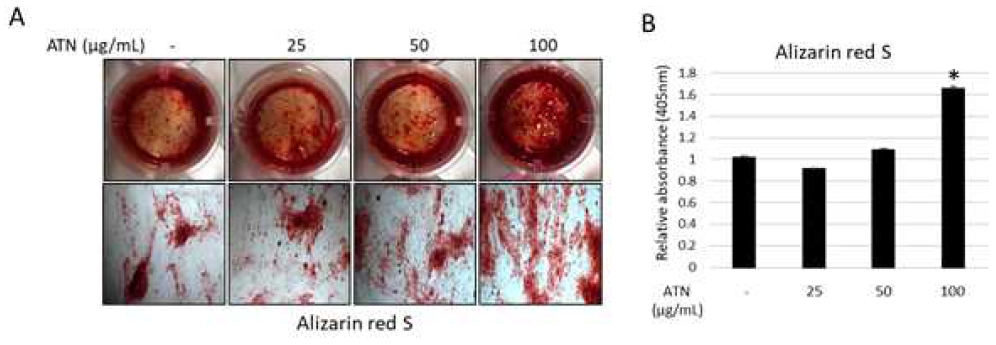
Effect of ATN on mineralized nodule formation of human periodontal ligament mesenchymal stem cells (hPDLSCs) (A) hPDLSCs were cultured for 3 weeks in osteogenic differentiation medium, either in the with or without ATN (100 μg/mL). Mineral nodule formation in hPDLSCs was determined using Alizarin Red S staining. (B) For measuring calcium content, the cells were destained and measured the absorption by spectrophotometry. *p<0.05, compared with the untreated control group (n=3).
4. ATN enhances the in vitro expression of osteo/odontoblastic differentiation markers in hPDLSCs
To investigate whether ATN induces osteo/odontoblastic differentiation of hPDLSCs, mRNA expression of related genes was accessed. Real-time PCR results revealed that mRNA expression levels of osteoblast- and odontoblast-associated markers, including ALP, COL1, OPN, DMP-1, and DSPP were significantly increased when hPDLSCs were treated with ATN for 3 weeks (Figure 4A). ATN-treated cells observed a significantly higher protein abundance of OCN, DSPP, DMP-1, and BSP compared to the control group (Figure 4B). Additionally, protein abundance of Runx2, a master transcriptional factor of osteogenic differentiation was evident in 100 μg/mL of ATN-treated group (Figure 4C). BSP promoter activity showed an increased tendency for over-expression of the OSX gene in HEK293 cells treated with ATN (100 μg/mL), compared with the control group (Figure 4D). These results indicate that ATN increases the osteo/odontoblastic differentiation of hPDLSCs.
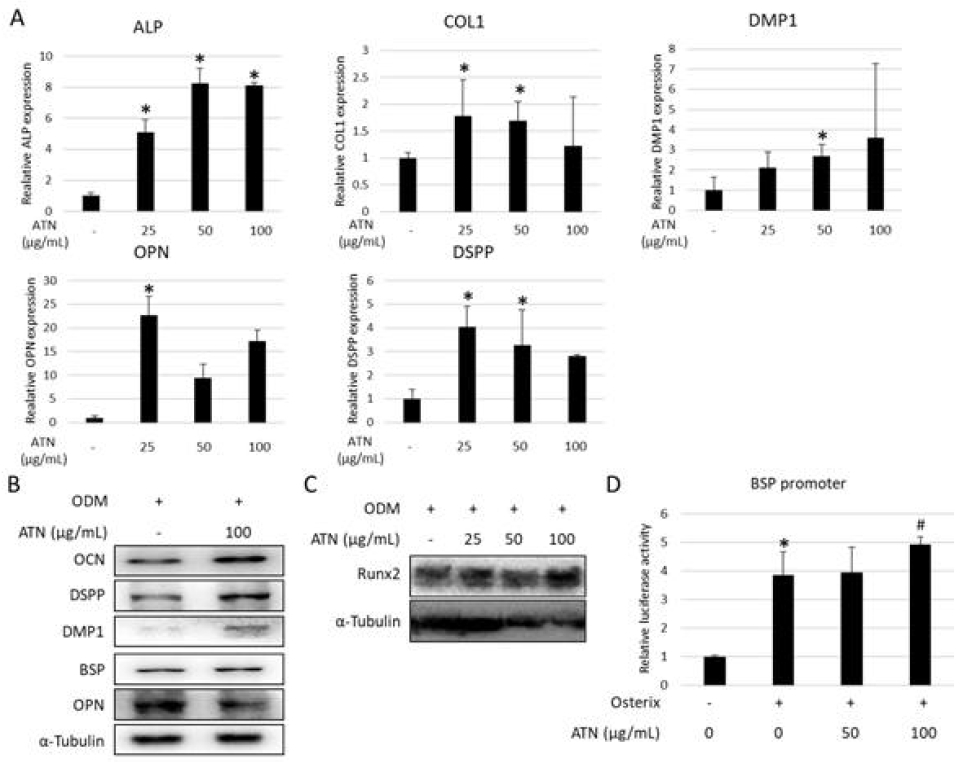
Effect of ATN on the expression of osteo/odontoblastic differentiation markers in human periodontal ligament mesenchymal stem cells (hPDLSCs) in vitro hPDLSCs were cultured for 3 weeks in osteogenic differentiation medium with or without indicated concentration of ATN (A) The mRNA expression of osteoblast- and odontoblast-associated genes were analyzed using real-time PCR. GAPDH was used as the internal control. (B, C). The protein abundances of osteoblast- and odontoblast-associated genes evaluated by western blotting. α-tubulin was used as the internal control. (D) The BSP promoter assay was performed in HEK293 cells (with OSX overexpression), either with or without ATN. *p<0.05, compared with the untreated control group, #p<0.05, compared with the osterix transfected group (n=3).
Discussion
The verification and validation of traditionally used natural remedies have led to the development of modern pharmaceuticals, and this validation helps provide the foundation for the design of novel drugs (21). While traditional medicinal substances might have a higher chance of being clinically safer than novel phytochemicals, the identification of their active ingredients remains challenging, owing to their varying composition based on their source of origin and their geographic location (22).
Dentin regeneration has been mainly studied using dental follicle stem cells (DFSCs), stem cells from human exfoliated deciduous teeth (SHEDs), and dental pulp stem cells (DPSCs) (23-25). While hPDLSCs have been previously studied for cementum and PDL regeneration (26, 27), a few studies have also reported the effects of hPDLSCs in a process of dentin regeneration. In a recent study comparing hPDLSCs with DFSCs, dentin regeneration was observed in a transplantation model with hPDLSCs in a treated dentin matrix (TDM) and examination of the harvested grafts showed that hPDLSCs formed dentin-like tissues, similar to those formed by DFSCs (28). However, the DFSC-generated dentin tissue structure appeared to be more complete than the hPDLSC formed tissue. The microenvironment of TDM appears to play a pivotal role in dentin regeneration in hPDLSCs (23). In this study, dentinogenesis-associated genes were used to evaluate ATN efficacy in dentin biogenesis. We found that the mRNA expression and protein abundance of odontoblastic differentiation-related genes such as DMP-1 and DSPP was upregulated (Figure 4A and B), suggesting the possibility of dentin regeneration using ATN. DMP-1 as a member of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family, is highly expressed in osteocyte and required for bone and dentin mineralization (29). Therefore, deficiency of DMP-1 could cases hypomineralization, owing to elevated FGF-23 production (30). DMP-1 was originally investigated to be specific to dentin, however it was also revealed to be detected and synthesized by osteoblast (31). Additionally, DSPP and its cleaved products, DSP and DPP, which were previously believed to be dentin-specific, have been recently shown to be expressed in bone, cementum, and some non-mineralized tissues (32). Nevertheless, many studies have suggested that these markers play important roles in dentin formation (33, 34).
In a present study, the influence of ATN on osteoblastic differentiation of hPDLSCs was evaluated. Alizarin red S staining indicated that calcium deposition increased after 3 weeks of incubation in osteogenic induction medium supplemented with ATN (Figure 3A and B). The mRNA expression of osteoblastic differentiation-related genes such as ALP, Col1, and OPN was upregulated, and western blot analysis with ATN showed increased protein abundances of OCN, BSP, and Runx2 (Figure 4A-C). Furthermore, HEK293 cells, with an OSX overexpression, showed a tendency of increased BSP promoter activity, compared to the control group (Figure 4D). Runx2 is a bone-specific transcription factor that plays a vital role in osteoblast differentiation (35, 36), since Runx-2 phosphorylation triggers the expression of various genes related in osteoblast differentiation (37). Similarly, ATN regulates osteoblast differentiation via phosphorylation. Additionally, OCN and BSP are osteoblast-specific markers present during the later stage of osteoblast differentiation. Therefore, upregulation of OCN and BSP expression indicates ATN-induced maturation of osteoblasts. In this study, the changes in osteoblastic differentiations-related gene expression suggested that ATN could promote osteoblastic differentiation of hPDLSCs. In addition, the dentinogenic roles of ATN were accessed in mice by implanting ATN-treated hPDLSCs at a site with no mineralization capacity (Data not shown). An inductive scaffold of TDM was used to achieve dentin regeneration using hPDLSCs in vivo. However, there was no remarkable difference between the control and ATN-treated groups, contradicting a previous study that stated PDLSCs could contribute for dentin tissue regeneration (28). Similar studies with DFSCs have reported that in vivo implantation immediately after seeding DFSCs to the TDM did not have the desired effect (32). In our study, since hPDLSCs were implanted into mice on the day post seeding in the TDM, it is possible that hPDLSCs can be transplanted without sufficient differentiation. Furthermore, multinucleated cells were observed around the TDM, and absorption of some dentin was suspected, which could be attributed to the death of hPDLSCs, owing to initial inflammatory reactions. Further in vivo elucidation is required to confirm the process of differentiation.
Conclusions
Combined with the results, we could conclude that ATN potentially promotes osteo/odontoblastic differentiation. It is difficult to assess the two effects separately because of the overlapping expression of genes involved in osteogenesis and odontogenesis.
Acknowledgments
This work has supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1F1A1040910, 2022R1C1C1002845).
References
-
Lee SH, Choi H, Kim H, Lee H, Sung YH, Kim SE, et al. Inhibitory effect of Angelicae Tenuissimae Radix on expressions of cyclooxygenase-2 and inducible nitric oxide synthase in mouse BV2 microglial cells. Neurol Res. 2010;32 Suppl 1:58-63.
[https://doi.org/10.1179/016164109X12537002794048]

-
Weeratunga P, Uddin MB, Kim MS, Lee BH, Kim TH, Yoon JE, et al. Interferon-mediated antiviral activities of Angelica tenuissima Nakai and its active components. J Microbiol. 2016;54(1):57-70.
[https://doi.org/10.1007/s12275-016-5555-4]

-
Bae N, Ahn T, Chung S, Oh MS, Ko H, Oh H, et al. The neuroprotective effect of modified Yeoldahanso-tang via autophagy enhancement in models of Parkinson’s disease. J Ethnopharmacol. 2011;134(2):313-22.
[https://doi.org/10.1016/j.jep.2010.12.016]

-
Ka MH, Choi EH, Chun HS, Lee KG. Antioxidative activity of volatile extracts isolated from Angelica tenuissimae roots, peppermint leaves, pine needles, and sweet flag leaves. J Agric Food Chem. 2005;53(10):4124-9.
[https://doi.org/10.1021/jf047932x]

-
Lee SM, Kim HJ, Jang YP. Chemometric classification of morphologically similar Umbelliferae medicinal herbs by DART-TOF-MS fingerprint. Phytochem Anal. 2012;23(5):508-12.
[https://doi.org/10.1002/pca.2348]

-
Jigden B, Wang H, Kyum Kim M, Kim YJ, Gyo In J, Yang DC. Authentication of the oriental medicinal plant Ligusticum tenuissimum (Nakai) Kitagawa (Korean Go-Bon) by multiplex PCR. Planta Med. 2010;76(6):648-51.
[https://doi.org/10.1055/s-0029-1240632]

-
Kim EO, Min KJ, Kwon TK, Um BH, Moreau RA, Choi SW. Anti-inflammatory activity of hydroxycinnamic acid derivatives isolated from corn bran in lipopolysaccharide-stimulated Raw 264.7 macrophages. Food Chem Toxicol. 2012;50(5):1309-16.
[https://doi.org/10.1016/j.fct.2012.02.011]

-
Li X, Wu D, Hu Z, Xuan J, Ding X, Zheng G, et al. The Protective Effect of Ligustilide in Osteoarthritis: An in Vitro and in Vivo Study. Cell Physiol Biochem. 2018;48(6):2583-95.
[https://doi.org/10.1159/000492701]

- Heyeraas KJ, Kvinnsland I. Tissue pressure and blood flow in pulpal inflammation. Proc Finn Dent Soc. 1992;88 Suppl 1:393-401.
-
Lin JJ, Du Y, Cai WK, Kuang R, Chang T, Zhang Z, et al. Toll-like receptor 4 signaling in neurons of trigeminal ganglion contributes to nociception induced by acute pulpitis in rats. Sci Rep. 2015;5: 12549.
[https://doi.org/10.1038/srep12549]

-
Zubizarreta-Macho A, Ferreiroa A, Agustin-Panadero R, Rico-Romano C, Lobo-Galindo AB, Mena-Alvarez J. Endodontic re-treatment and restorative treatment of a dens invaginatus type II through new technologies. J Clin Exp Dent. 2019;11(6):e570-e6.
[https://doi.org/10.4317/jced.55840]

-
Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809-20.
[https://doi.org/10.1016/S0140-6736(05)67728-8]

-
Hu L, Liu Y, Wang S. Stem cell-based tooth and periodontal regeneration. Oral Dis. 2018;24(5):696- 705.
[https://doi.org/10.1111/odi.12703]

-
Liu KN, Huang Z, Chen ZB, Han B, Ouyang XY. Treatment of periodontal intrabony defects using bovine porous bone mineral and guided tissue regeneration with/without platelet-rich fibrin: a randomized controlled clinical trial. J Periodontol. 2021;92(11):1546-53.
[https://doi.org/10.1002/JPER.20-0860]

-
Chen FM, Sun HH, Lu H, Yu Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials. 2012;33(27):6320-44.
[https://doi.org/10.1016/j.biomaterials.2012.05.048]

-
Bright R, Hynes K, Gronthos S, Bartold PM. Periodontal ligament-derived cells for periodontal regeneration in animal models: a systematic review. J Periodont Res. 2015;50(2):160-72.
[https://doi.org/10.1111/jre.12205]

-
Seo BM, Miura M, Gronthos S. Investigation of multipotent postnatal stem cells from human periodontal ligament. (vol 364, pg 149, 2004). Lancet. 2004;364(9447):1756-.
[https://doi.org/10.1016/S0140-6736(04)16627-0]

-
Zhu WJ, Liang M. Periodontal Ligament Stem Cells: Current Status, Concerns, and Future Prospects. Stem Cells Int. 2015;2015:972313.
[https://doi.org/10.1155/2015/972313]

-
Yadav SK, Khan G, Bansal M, Thokala S, Bonde GV, Upadhyay M, et al. Multiparticulate based thermosensitive intra-pocket forming implants for better treatment of bacterial infections in periodontitis. Int J Biol Macromol. 2018;116:394-408.
[https://doi.org/10.1016/j.ijbiomac.2018.04.179]

-
Hwang JW, Park WJ, Han Y. Asarylaldehyde enhances osteogenic differentiation of human periodontal ligament stem cells through the ERK/p38 MAPK signaling pathway. Biochem Biophys Res Commun. 2021;545:27-32.
[https://doi.org/10.1016/j.bbrc.2021.01.053]

-
Bibi Sadeer N, Sinan KI, Cziaky Z, Jeko J, Zengin G, Jeewon R, et al. Towards the Pharmacological Validation and Phytochemical Profiling of the Decoction and Maceration of Bruguiera gymnorhiza (L.) Lam.-A Traditionally Used Medicinal Halophyte. Molecules. 2022;27(6)2000.
[https://doi.org/10.3390/molecules27062000]

- Li GH, Chen S, Zhang J, Wang YS, Cheng JT, Liu A. [Explore a method for formulation of limit standard of toxic components in traditional Chinese medicine: aristolochic acid as a case study]. Zhongguo Zhong Yao Za Zhi. 2017;42(4):800-4.
-
Li R, Guo WH, Yang B, Guo LJ, Sheng L, Chen G, et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials. 2011;32(20):4525-38.
[https://doi.org/10.1016/j.biomaterials.2011.03.008]

-
Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8(3):191-9.
[https://doi.org/10.1111/j.1601-6343.2005.00331.x]

-
Jin B, Choung PH. Recombinant Human Plasminogen Activator Inhibitor-1 Accelerates Odontoblastic Differentiation of Human Stem Cells from Apical Papilla. Tissue Eng Part A. 2016;22(9-10):721-32.
[https://doi.org/10.1089/ten.tea.2015.0273]

-
Jin H, Choung HW, Lim KT, Jin B, Jin C, Chung JH, et al. Recombinant Human Plasminogen Activator Inhibitor-1 Promotes Cementogenic Differentiation of Human Periodontal Ligament Stem Cells. Tissue Eng Part A. 2015;21(23-24):2817-28.
[https://doi.org/10.1089/ten.tea.2014.0399]

-
Park JY, Park CH, Yi T, Kim SN, Iwata T, Yun JH. rhBMP-2 Pre-Treated Human Periodontal Ligament Stem Cell Sheets Regenerate a Mineralized Layer Mimicking Dental Cementum. Int J Mol Sci. 2020;21 (11):3767.
[https://doi.org/10.3390/ijms21113767]

-
Tian Y, Bai D, Guo W, Li J, Zeng J, Yang L, et al. Comparison of human dental follicle cells and human periodontal ligament cells for dentin tissue regeneration. Regen Med. 2015;10(4):461-79.
[https://doi.org/10.2217/rme.15.21]

- Harris SE, Gluhak-Heinrich J, Harris MA, Yang W, Bonewald LF, Riha D, et al. DMP1 and MEPE expression are elevated in osteocytes after mechanical loading in vivo: theoretical role in controlling mineral quality in the perilacunar matrix. J Musculoskelet Neuronal Interact. 2007;7(4):313-5.
-
Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310-5.
[https://doi.org/10.1038/ng1905]

-
D’Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB, et al. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res. 1997;12(12):2040-9.
[https://doi.org/10.1359/jbmr.1997.12.12.2040]

-
Prasad M, Butler WT, Qin C. Dentin sialophosphoprotein in biomineralization. Connect Tissue Res. 2010;51(5):404-17.
[https://doi.org/10.3109/03008200903329789]

-
Quispe-Salcedo A, Ida-Yonemochi H, Nakatomi M, Ohshima H. Expression patterns of nestin and dentin sialoprotein during dentinogenesis in mice. Biomed Res. 2012;33(2):119-32.
[https://doi.org/10.2220/biomedres.33.119]

-
Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, et al. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278(27):24874-80.
[https://doi.org/10.1074/jbc.M303908200]

-
Choi YH, Han Y, Jin SW, Lee GH, Kim GS, Lee DY, et al. Pseudoshikonin I enhances osteoblast differentiation by stimulating Runx2 and Osterix. J Cell Biochem. 2018;119(1):748-57.
[https://doi.org/10.1002/jcb.26238]

-
Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423(6937):349-55.
[https://doi.org/10.1038/nature01660]

-
Ren D, Wei F, Hu L, Yang S, Wang C, Yuan X. Phosphorylation of Runx2, induced by cyclic mechanical tension via ERK1/2 pathway, contributes to osteodifferentiation of human periodontal ligament fibroblasts. J Cell Physiol. 2015;230 (10):2426-36.
[https://doi.org/10.1002/jcp.24972]