Antifungal effect and characterization of denture PMMA impregnated with chitosan
Abstract
In this study, we proposed a modified polymethylmethacrylate (PMMA) denture acrylic impregnated with chitosan as a novel antimicrobial denture base material. We fabricated the samples of chitosan-PMMA denture acrylic (CPD) into four types (1.0 , 3.0 and 5.0 % and 0 %; control) according to the mass fraction (w%) of chitosan blended to pristine PMMA powder with methylmethacrylate (MMA) liquid by the ratio of 1.7 g/ml. For mechanical evaluations, surface morphology, degree of conversion, flexural strength and color changes were tested. The antifungal effects were evaluated in vitro as a number of viable cells (CFU; colony forming unit) in retrieved fungal suspension on samples after 24 hour incubation. A significantly reduced CFU was exhibited at 5.0 % CPD (p < 0.05) by the 40.9 ± 2.1 % inhibitory effect. CPD exhibited the similar microscopic surface texture and no statistical differences (p > 0.05) of the degree of conversion and flexural strength values as compared to pristine PMMA. Color differences were detected at 3.0 and 5.0 % of CPD by the reference value of 2.69. Within the limitation of present study, CPD could be a possible intrinsic antifungal denture material with the proper mechanical characters for a denture biomaterial. For clinical application, future studies including color stability, anti-adherent mechanism and the long-term effect were still required.
초록
키토산이 투여된 의치상 레진 (CPD: chitosan-PMMA denture acrylic)의 항균특성 및 물성을 평가하기 위해 키토산 분말을 PMMA 분말에 0 (대조군), 1.0, 3.0 및 5.0 w% (질량 분율)로 첨가한 후 혼수비 1.7g/ml로 MMA용액과 중합하였다. 항균특성은 Candida albicans (ATCC 14053) 희석 균액 100 μL를 시편표면에 접종하고 24 시간 배양 후 회수 된 진균 현탁액의 생균수 (CFU; colony forming unit)로 평가하였고 키토산 분말첨가에 따른 의치의 물성변화는 중합전환율, 굴곡 강도 및 색조안정성을 통하여 측정하였다. 5.0 % CPD에서 대조군에 대한 유의성 있는 진균 부착 억제율 (40.9 ± 2.1 %)이 관찰되었고 키토산 첨가에 따른 중합전환율, 굴곡 강도 수치의 통계적 차이는 나타내지 않았으나 (p=0.05) 3.0 w% 이상 투여 군들에서 농도비례적으로 허용 색조변화 기준치 (ΔE =2.69)를 초과하였다. 본 연구의 키토산-의치상 레진 복합체는 적절한 물성과 내재적 항균특성을 가진 치과생체재료로서의 가능성을 도출하였고 임상 적용을 위하여 색조 안정성 개선을 포함한 명확한 항균작용기전 그리고 장기 효과 등의 연구들이 계속 진행되어야 할 것으로 사료된다.
Keywords:
Antifungal effects, Chitosan, Color stability PMMA denture acrylic, Mechanical property키워드:
항진균효과, 키토산, Color stability PMMA denture acrylic, 기계적 성질Ⅰ. INTRODUCTION
Denture try-on inevitably compromises salivary flow related with hygienic effect and encourages biofilm formations on both the prosthetic surface and adjacent mucosa (Yildirim et al., 2005). Chronic denture stomatitis is an erythematous pathogenic condition of denture-bearing mucosa and one of the major etiological factors in this pathogenesis is the presence of numerous yeasts, usually Candida albicans (C. albicans) on the fitting surface of the denture (Boscato et al., 2009; Paranhos et al., 2009). Despite the use of antifungal agents to cure denture stomatitis, infection often recurs and drug tolerance has been observed in majority of cases (Chandra et al. 2001). Chemical-based oral disinfectants such as sodium hypochlorite or glutaraldehyde has been prescribed, however, their bleaching agents may interfere with the esthetics of the prostheses or might be irritant to oral mucosa due to their toxic vapors (Chassot et al., 2006). Microwave disinfection of denture base in a 60 Hz for 5 minutes was suggested to kill colonized fungi, however, the repeated irradiations significantly affected the hardness of material itself (Dixon et al., 1999). Studies for the antimicrobial denture materials incorporated with silver nanoparticles (Wady et al., 2012; Monteiro et al., 2012; Nam et al., 2012) have been reported, the significant discolorations of nanocomposites discouraged the clinical application (Chladek et al., 2011; Nam, 2014). To overcome denture-related inflammatory complications, performative and latent antimicrobial denture bases are highly requested and mandatory (Saito et al., 1997), however, they have not been available in market yet.
Recently, higher interests are focusing on synthesis of biomaterials holding antimicrobial properties in dental field. Chitosan, a polysaccharide biopolymer derived from naturally occurring chitin, displays unique polycationic, chelating, and bacterial or fungal inhibiting properties due to the presence of active amino and hydroxyl functional groups (Wang et al., 2006; Yi et al., 2005). Due to its antibacterial property, chitosan has been blended with other polymers such as alginate, hydroxyapatite, hyaluronic acid, calcium phosphate, PMMA (Hu et al., 2003). Chitosan also has a high resistance to heat due to its intramolecular hydrogen bonds (Onishi and Machida, 1999) and PMMA is strongly attached onto the chitosan surface (Wieckiewicz et al., 2017), therefore, PMMA denture might be appropriate as a possible carrier to apply chitosan to the oral mucosa.
Present study demonstrates the synthesis of a modified PMMA denture base incorporated with chitosan and determines whether the modified denture acrylic exhibits antifungal properties. Moreover, we also examine whether the chitosan additives influence the physico-mechanical properties of the pristine PMMA denture. The hypothesis is that chitosan-PMMA denture acrylic complex creates an antifungal activity and result in stable physical properties for the clinical application.
Ⅱ. MATERIALS AND METHODS
1. Fabrication of chitosan-PMMA Denture acrylic (CPD)
The specimens for test were prepared as following. Chitosan (low molecular type, 448869, Sigma–Aldrich Co. USA) with degree of deacetylation and molecular weights are 75% and 50,000 - 190,000 Da respectively, was used without further purifications (Figure 1). Primarily, chitosan powders were homogenously blended into pristine PMMA powder (Vertex-SCⓇ, Vertex-Dental B.V., Netherlands) according to different mass ratios of 1.0 , 3.0 and 5.0 % [weight (w)%] respectively. Unmodified PMMA group was designated as the control (0 % of chitosan). Blended powders (chitosan-PMMA) were then mixed with MMA (methyl methacrylate: Vertex-SCⓇ, Vertex-Dental B.V., Netherlands) liquid at designated powder/liquid ratio of 1.7 g/ml. When mixtures became a dough-staged, they were packed into two type of custom moulds by disc (20 mm diameter × 2 .0 mm depth) and rectangular (25 mm × 2 mm × 2 mm) specifications. The mixtures in moulds were sandwiched between two glass plates under 10 kg static pressure then cured following the manufacturer’s instructions. Cured samples were trimmed and immersed for 120 hours in sterilized distilled water to leach excess residual monomer then finished for 1 hour in distilled water using ultrasonic cleaner. The morphological surface characterization of CPD was examined by SEM (Scanning Electron Microscopy; S-4200 FESEM, Hitachi, Japan).
2. Mechanical evaluations
The DC of samples (n = 40) were determined by Raman spectroscopy (Jobin-Yvon Horiva HR800, Japan) with a CCD 3000 (V) detector and Labspec 4 .01 software. He-Ne ion laser (λ = 632.8 nm) was used as the excitation source. The laser power and confocal size were 15 mW and 400 μm, respectively. The DC (%) of monomer-to-polymer was calculated by comparison of the absorbance ratio using a standard baseline technique of the C = C peak from the methacrylate group at 1637 cm-1 to that of the unchanging C = O peak from the ester group at 1724 cm-1 (a reference peak) before and after polymerization. By taking the ratio between the two absorbance, the fraction of unreacted double bonds could be calculated from the formula: DC (%) = [1 – w% methacrylate groups in cured resin / w% methacrylate groups in uncured resin] × 100 % .
To evaluate the flexural strength of CPD, rectangular samples (n = 40) were fabricated according to ISO 4049:2000(E). The load was applied perpendicular to the center of the specimen at a crosshead speed of 1 mm/min with a universal testing machine (Model 4200, Instron Inc., USA).
Color stability was measured with disc specimens (n = 20) by using spectrophotometer Color-readerⓇ (Minolta CR-10, Japan) at the time of 24 and 168 hours from the onset of curing process. The mean of three reading was recorded and the value of each specimen was calculated with use of the CIE LAB scale recommended by Commission Internationale de l’Eclairage. Means were calculated and total color change (ΔE*) of CPD were calculated using the followed relationship:
ΔE*= [(ΔL*)2+(Δa*)2+(Δb*)2]½, where ΔL*=L1-L0, Δa*= a1-a0, Δb*= b1-b0
(L1, a1, b1 : chitosan impregnated, L0, a0, b0 : control)
3. Antifungal assay
C. albicans (ATCC 14053) strain was selected and grown in Schaedler broth. After incubating fungal cells at 37°C overnight, optical density of fungal suspension at 600 nm was adjusted to 1.0 using a spectrophotometer. The suspension was diluted with phosphate-buffered saline (pH 7.4) to 1:100 and suspended to final concentration of 1.0 × 107 cells/mL. Disc samples were sterilized with ethylene oxide gas for 24 hours to ensure the initial sterility and then placed on multi-well culture plate plate (CostaⓇ, Corning, New York, USA) with a 22.1 mm diameter. Initial microbial suspensions (100 μL) in 1.0 ml of Sabouraud broth were inoculated to each well and incubated at 37°C. After 24 hour-incubating, suspension (100 μL) was withdrawn, viable cells (CFU; colony forming unit) in the suspension were determined by using the spread plate method at a level of detection within 500 CFU per plate through the serial dilution.
4. Statistics
The data were statistically analyzed using one-way ANOVA and the multiple comparisons Scheffe’s test at the significance level of 0.05.
Ⅲ. RESULT
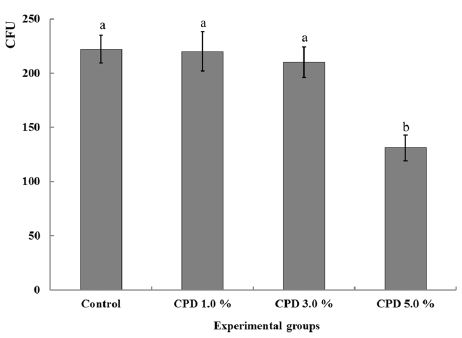
The surface textures of 5.0 % CPD were similar to those of the control (× 1000) in roughness and porosity (Figure 2). The mean DC (%) and flexural strength (MPa) with standard deviations for tested samples are given in Table 1. The highest mean DC value was observed in control (73.2 %), however, all of CPD groups (1.0 – 5.0 %) did not differ statistically (P > 0.05) to control group with values ranged 67.4 to 71.2 %. In flexural strength, CPD groups also expressed no significant differences (P > 0.05) from control with values ranged 72.3 to 76.7 MPa. Between CPD groups, no statistically significant difference was observed in both mechanical tests respectively (P > 0.05). The mean values of color difference for of CPD are shown in table 2. ΔE* were detected as 2.1 - 2.4 (1.0 % CPD), 5.2 - 5.6 (3.0 % CPD), 7.3 - 7.9 (5.0 % CPD) at 24 and 168 hours respectively. The higher chitosan added, the significantly greater ΔE* were expressed (P < 0.05) and no statistically significant difference was observed between two intervals (P > 0.05). The antifungal effects of CPD were demonstrated as CFU after 24 hours of incubation (Table 3). When compared with control, a significant fungal inhibition was shown at CPD 5.0 % with the reduction rate 40.9 % (p < 0.05) and CPD of 1 .0 % and 3 .0 % did not exhibited significant antifungal effects (p > 0.05) (Fig. 4).

Comparative SEM images of control (left) and 5.0 % CPD (right), microscopic surface texture of CPD is similar to those of the control (x 1.000)

Photographs of color changings according to chitosan doses after 168 hour from onset of curing. When compared to pristine, the colors of CPD become paler as the concentration of chitosan increased
Ⅳ. DISCUSSION
It is important for CPD to evaluate the conversion degree relating to the amount of residual monomer as chitosan added. Chitosan particles could act as heterogeneous nuclei or impurities and this may influence resin curing processes or jeopardize PMMA matrix which eventually compromise the sufficient flexural strength to resist fracture. In this study, DC of denture PMMA was not influenced by chitosan impregnations and liquid monomer could diffuse to reactive radical ends of polymer regardless of chitosan maximally blended by 5.0 %. Some reports showed that combining with additives, such as fibers (Narva et al., 2005) and whiskers (Niu et al., 2010), improved the mechanical properties of polymers. Other reported the addition of silver zinc zeolite results in a significant decrease in the flexural and impact strengths of denture acrylic (Casemiro et al. (2008). Usually, the manufacturer determines the optimal P/L ratio to satisfy its minimal flexural strength by more than 60 MPa. Though values of flexural strength of CPD were decreased as chitosan dose increased, chitosan played non-negative effect in physical function of PMMA. All of CPD samples exhibited no significant differences from control expressing minimally 72.3 MPa in 5.0 % (Table 1). Color stability is another important clinical behavior for denture base since it may provide cosmetic service of this material. Despite many studies determining the efficacy of resin composites loading various antimicrobial materials (Ahn et al., 2009; Wady et al., 2012; Monteiro et al., 2012), there have been little information regarding the color stability influenced by additives. Generally, if color difference (ΔE*) were significantly greater than 2.69, the perceptibility threshold (Chang et al., 2009), the clinical applications should be limited. When compared to control, 1.0 % CPD groups were solely detected within the perceptibility threshold by 2.1 to 2.4 at the time of 24 and 168 hours elapsed from the onset of curing. CPD become paler and slight darker as the concentration of chitosan increased (Fig. 3) and an early discoloration within 24 hours from the onset of curing were expressed by 5.2 and 7.3 in 3.0 % and 5.0 % respectively (Table 2). Oxidative reaction or plasmon effect (Bohren and Huffman, 2007) by metal nanoparticles is known to influence the color instability of resin (Chladek et al., 2011; Nam, 2014), unique color of chitosan powder might contribute to this discoloration. Further studies are still needed to stabilize the color through alternative blending or chemical regulation of chitosan when added into the denture PMMA. In antifungal assay, a small volume of fungal suspension (100 μL) was used because immersing samples in a large suspension volume could not reproduce in denture fitting on gingival mucosa (Baehni and Takeuchi, 2003). 5.0 % CPD statistically inhibited biofilm formation by 40.9 % on its surface compared to control groups (Table 3), providing a promising new strategy for combating denture stomatitis. The knowledge have already been acquired for the antimicrobial effect of chitosan against fungi, (Wang et al., 2006; Yi et al., 2005), however, few information about the antimicrobial effect after their incorporation to solid acrylic resin bulk. Furthermore, the mechanism of antimicrobial denture composite has not been fully determined yet. Chitosan is reported to exhibit a strong interaction with negative charges on the bacterial cell surface and it showed better antibacterial activity with higher concentration in the biofilm prevention and susceptibility assays (Peng et al., 2011). Plastic surface generally possesses variable negative surface charges; similarly, all living bacterial cells (including yeasts) possess a net negative surface charge (Klotz et al., 1985). It could be speculated that chitosan may alter the physicochemical interactions or modify the polarity of PMMA surface which induce the anti-adherent power against the negatively charged fungal cell. Nevertheless, the present results cannot jump to the conclusion, because the experiment was an in vitro pattern, short-term analysis with a sole fungal strain selection. Further investigations including unknown interfacial factors, in vivo tests, tests of other strains, and long-term observations are needed to clarify the antifungal mechanism.

Results of degree of conversion and flexural strength. Data were expressed as mean values with standard deviation (SD)

The color stability of CPD as to control at the time of 24 and 168 hours elapsed from the onset of curing process. ΔE* means the color difference from control
Ⅴ. CONCLUSION
In present study, self-polymerizing PMMA denture with 5.0 w% chitosan powders significantly inhibited the fungal adherence with proper physico-mechanical characters except color stability as compared to pristine PMMA. For clinical applications of chitosan-PMMA denture acrylic complex as a new bio-denture material, color stability, long-term antifungal effect and the verified antimicrobial mechanism still need to be investigated.
References
-
Ahn, SJ, Lee, SJ, Kook, JK, Lim, BS, (2009), Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles, Dent Mater, 25, p206-213.
[https://doi.org/10.1016/j.dental.2008.06.002]

-
Baehni, PC, Takeuchi, Y, (2003), Anti-plaque agents in the prevention of biofilmassociated oral diseases, Oral Dis, 9, p23-29.
[https://doi.org/10.1034/j.1601-0825.9.s1.5.x]

-
Boomi, P, Prabu, HG, Mathiyarasu, J, (2013), Synthesis and characterization of polyaniline/Ag-Pt nanocomposite for improved antibacterial activity, J Colloids Surf B Biointerfaces, 103, p9-14.
[https://doi.org/10.1016/j.colsurfb.2012.10.044]

- Bohren, CF, Huffman, DR, (2007), Absorption and Scattering of Light by Small Particles, New York, Wiley, p515.
-
Boscato, N, Radavelli, A, Faccio, D, (2009), Biofilm formation of Candida albicans on the surface of a soft denture-lining material, Gerodontology, 26, p210-213.
[https://doi.org/10.1111/j.1741-2358.2008.00267.x]

-
Casemiro, LA, Gomes, Martins CH, Pires-de-Souza, C, Panzeri, H, (2008), Antimicrobial and mechanical properties of acrylic resins with incorporated silver-zinc zeolite - part I, Gerodontology, 25, p187-194.
[https://doi.org/10.1111/j.1741-2358.2007.00198.x]

-
Chang, J, Da, Silva JD, Sakai, M, Kristiansen, J, Ishikawa-Nagai, S, (2009), The optical effect of composite luting cement on all ceramic crowns, J Dent, 37, p937-943.
[https://doi.org/10.1016/j.jdent.2009.07.009]

-
Chladek, G, Mertas, A, Barszczewska-Rybarek, I, Nalewajek, T, Zmudzki, J, Krol, W, Lukaszczyk, J, (2011), Antifungal activity of denture soft lining material modified by silver nanoparticles-a pilot study, Int J Mol Sci, 12, p4735-4744.
[https://doi.org/10.3390/ijms12074735]

-
Chandra, J, Mukherjee, PK, Leidich, SD, Faddoul, FF, Hoyer, LL, Douglas, LJ, Ghannoum, MA, (2001), Antifungal resistance of candidal biofilms formed on denture acrylic in vitro, J Dent Res, 80, p903-908.
[https://doi.org/10.1177/00220345010800031101]

-
Chassot, A, Poisl, MI, Samuel, SM, (2006), In vivo and in vitro evaluation of the efficacy of a peracetic acid-based disinfectant for decontamination of acrylic resins, Braz Dent, 17, p117-121.
[https://doi.org/10.1590/s0103-64402006000200006]

-
Dixon, DL, Breeding, LC, Faler, TA, (1999), Microwave disinfection of denture base materials colonized with Candida albicans, J Prosthet Dent, 81, p207-214.
[https://doi.org/10.1016/s0022-3913(99)70250-7]

- Hu, SG, Jou, CH, Yang, MC, (2003), Yang Protein adsorption, fibroblast activity and antibacterial properties of poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) grafted with chitosan and chitooligosaccharide after immobilized with hyaluronic acid, Biomaterials, 24, p2685-2693.
- Klotz, SA, Drutz, DJ, Zajic, JE, (1985), Factors governing adherence of Candida species to plastic surfaces, Infect Immun, 50, p97-101.
-
Monteiro, DR, Gorup, LF, Takamiya, AS, de Camargo, ER, Filho, AC, Barbosa, DB, (2012), Silver distribution and release from an antimicrobial denture base resin containing silver colloidal nanoparticles, J Prosthodont, 21, p7-15.
[https://doi.org/10.1111/j.1532-849x.2011.00772.x]

-
Nam, KY, (2014), Antifungal effect and characterization of denture PMMA nanocomposite containing gold, platinum and silver nanoparticles, Korean J Dent Mater, 41, p67-75.
[https://doi.org/10.14815/kjdm.2014.41.1.67]

-
Nam, KY, Lee, CH, Lee, CJ, (2012), Antifungal and physical characteristics of modified denture base acrylic incorporated with silver nanoparticles, Gerodontology, 29, pe413-419.
[https://doi.org/10.1111/j.1741-2358.2011.00489.x]

-
Narva, KK, Lassila, LV, Vallittu, PK, (2005), The static strength and modulus of fiber reinforced denture base polymer, Dent Mater, 21, p421-428.
[https://doi.org/10.1016/j.dental.2004.07.007]

-
Niu, L, Fang, M, Jiao, K, Tang, L, Xiao, Y, Shen, L, Chen, JH, (2010), Tetrapod-like zinc oxide whisker enhancement of resin composite, J Dent Res, 89, p746-750.
[https://doi.org/10.1177/0022034510366682]

-
Onishi, H, Machida, Y, (1999), Biodegradation and distribution of water-soluble chitosan in mice, Biomaterials, 20, p175-182.
[https://doi.org/10.1016/s0142-9612(98)00159-8]

- Paranhos, HF, Silva-Lovato, CH, de Souza, RF, Cruz, PC, de Freitas-Pontes, KM, Watanabe, E, Ito, IY, (2009), Effect of three methods for cleaning dentures on biofilms formed in vitro on acrylic resin, J Prosthodont, 18, p427-431.
-
Peng, ZX. Tu B, Shen, Y, Du, L, Wang, L, Guo, SR, Tang, TT, (2011), Quaternized chitosan inhibits icaA transcription and biofilm formation by Staphylococcus on a titanium surface, Antimicrob Agents Chemother, 55, p860-866.
[https://doi.org/10.1128/aac.01005-10]

-
Saito, T, Takatsuka, T, Kato, T, Ishihara, K, Okuda, K, (1997), Adherence of oral streptococci to an immobilized antimicrobial agent, Arch Oral Biol, 42, p539-545.
[https://doi.org/10.1016/s0003-9969(97)00054-x]

-
Wady, AF, Machado, AL, Zucolotto, V, Zamperini, CA, Berni, E, Vergani, CE, (2012), Evaluation of Candida albicans adhesion and biofilm formation on a denture base acrylic resin containing silver nanoparticles, J Appl Microbiol, 112, p1163-172.
[https://doi.org/10.1111/j.1365-2672.2012.05293.x]

-
Wang, B, Chen, K, Jiang, S, Reincke, F, Tong, W, Wang, D, Gao, C, (2006), Chitosan-mediated synthesis of gold nanoparticles on patterned poly(dimethylsiloxane) surfaces, Biomacromolecules, 7, p1203-1209.
[https://doi.org/10.1021/bm060030f]

-
Wieckiewicz, M, Boening, KW, Grychowska, N, Paradowska-Stolarz, (2017), A Clinical application of chitosan in dental specialities, Mini Rev Med Chem, 17, p401-409.
[https://doi.org/10.2174/1389557516666160418123054]

- Yi, H, Wu, LQ, Bentley, WE, Ghodssi, R, Rubloff, GW, Culver, JN, Payne, GF, (2005), Biofabrication with chitosan, Biomacromolecules, 6, p2881-2894.
-
Yildirim, MS, Hasanreisoglu, U, Hasirci, N, Sultan, N, (2005), Adherence of Candida albicans to glow-discharge modifies acrylic denture base polymers, J Oral Rehabil, 32, p518-525.
[https://doi.org/10.1111/j.1365-2842.2005.01454.x]