Comparison of three methods for determination of the degree of conversion in commercial light-cured composites
본 실험의 목적은 DSC와 FTIR을 통해 결정한 복합 레진의 전환율을 비교하는 것이다. 다섯 가지 시판 중인 복합 레진을 사용하였다. FTIR을 이용하여 여러 농도로 희석시킨 methyl methacrylate (MMA)의 검정곡선을 구하였다. Weight percent of methacrylate groups (WPMG) 방법을 이용하여 레진의 중합 전후의 WPMG 변화를 계산하였다. 또한 DSC 방법을 이용하여 이론적인 발열값 (exotherm)과 실험적인 발열값을 결정하였다. 다섯 가지 복합 레진에 대하여 internal standard, WPMG, DSC 방법을 통해 전환율을 계산하였다. Internal standard와 WPMG 방법을 사용한 경우 각 복합 레진에서 유의한 차이가 관찰되지 않았으나 (p>0.05), DSC 방법을 사용한 경우 유의하게 낮은 전환율 값이 얻어졌다 (p<0.05). FTIR로 복합 레진의 전환율을 측정 시 internal standard가 적당하지 않은 경우 WPMG 방법을 적용할 수 있을 것으로 생각된다.
Keywords:
Resin composite, Degree of conversion, DSC, FTIR spectroscopy서 론
Degree of conversion (DC) is one of the important factors that affect clinical performance of dental resin composites (Imazato et al., 2001). The physical and mechanical properties of photo-cured composites are directly influenced by the level of conversion attained during polymerization (Moraes et al. 2008).
The methodology of determining degree of conversion in dental composite materials by means of Fourier transform infrared (FTIR) spectrometer has been successfully used to determine DC. An internal standard method is the absorption of a functional group that does not enter into reaction during curing (e.g., aromatic C=C). The early commercial composite mostly contained Bis-GMA (2,2-bis[4-(3-methacryloyloxy-2-hydroxypropoxy) phenyl] monomer. The aromatic C=C contained in Bis-GMA-based resins was successfully used as internal reference peak for ratioing the aliphatic C=C since it is stable in the matrix resin during reaction (Yoshida and Greener, 1993). Afterwards, the urethane (N-H) and carbonyl (C=O) (Yoshida and Greener, 1993) were also used as internal references with the appearance of UDMA (urethane dimethacrylate) monomer. This method has been successfully and mainly used to determine DC of composites.
However, as the development of dental resins, new monomers which do not contain internal reference to serve as a standard, may be appeared. A new method to determine DC having no internal standard was developed by Loshaek and Fox (1953), and verified by Rueggeberg (1994) and Bartoloni et al. (2000). This method is based on the assumption that the gravimetric form of methacrylate group (H2C=C(CH3)C=O, molecular weight: 69.081) can be used for concentration measurements. DC of monomers is expressed as a proportion in weight percent of the methacrylate groups (WPMG) remaining after polymerization relative to the total number of C=C in the uncured resins. It is not influenced by changes in density of the resin as it cures (Rueggeberg, 1994). The WPMG method includes the following four steps: (1) the establishment of the absorbance vs. concentration (moles C=C/mL) calibration curve of methyl methacrylate (MMA); (2) the absorbance analysis of uncured composite resins and the calculation of C=C/mL using the calibration curve (WPMGu); (3) the transmittance analysis of cured composite resins (WPMGc); and (4) the calculation of final DC using the ratio of WPMGc to WPMGu (Ha et al., 2011).
Differential scanning calorimetry (DSC) is a convenient tool for the analysis of the polymerization behavior of dental resin monomers. It is a direct method that analyses the extent of polymerization based on the assumption that heat generated during resin polymerization is proportional to the percentage or concentration of reacted monomers (Cadenaro et al., 2005). The extent and rate of the polymerization of functional vinyl monomers can be analyzed by measuring the heat value of the exothermal peak area, enabling determination of degree of polymerization.
Applying WPMG and DSC methods to calculating DC, it is necessary to determine the aliphatic C=C concentration of the uncured material. However, it is nearly impossible to calculate the amount of aliphatic C=C of commercial composite because it would be necessary to know the exact amount of each monomers in each material, which is not provided by the manufacturers. In this case, it is necessary to build a calibration curve. The calibration curve has been successfully used to determine the DC of liquid resin monomers (Rueggeberg, 1994), adhesive (Bartoloni et al., 2000) and denture base materials (Cadenaro et al., 2005) employing WPMG method. However, a limited numbers of studies applied this curve on WPMG and DSC methods to calculate DC of commercial composites.
The purpose of this study was to determine the degree of conversion of commercial composites by DSC and FTIR. The null hypothesis was that calibration curve could be applied to WPMG and DSC methods to determine the DC of commercial composites
MATERIALS AND METHODS
Composites
Five commercial composites were used in this study, and their compositions are listed in Table 1.

Weight percent of methacrylate groups in uncured monomers and theoretical amount of heat evolved during polymerization of 1 g composite
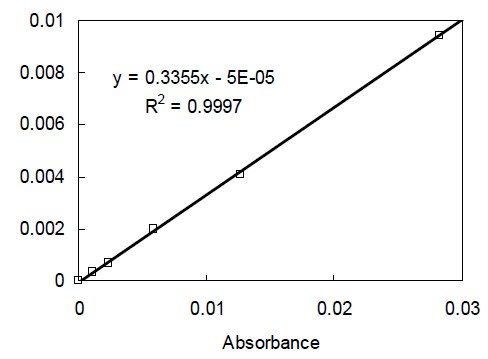
Solutions of dilution of MMA (Junsei Chemical Co., Ltd., Tokyo, Japan) with spectroscopic grade hexane were prepared. The molecular weight (100.12 g/mol) and density (0.94 g/ml) of MMA were used to calculate the number of moles of C=C per ml of each of the standard solutions (Rueggeberg, 1994; Bayne, 2005). The infrared spectrum of each solution was obtained using a Fourier transform infrared (FTIR) spectroscope (IRPrestige-21, Shimadzu, Tokyo, Japan) with an attenuated total reflectance unit (MIRacle, Pike Technologies, Inc., Madison, WI, USA) under the experimental conditions (gain: 1, 8 scans, 2 cm-1 resolution). The height of the absorbance peak of the aliphatic C=C at 1636 cm-1 was determined by baseline technique (Rueggeberg, 1994). Linear regression analysis was performed on the data to produce the mathematical relationship between the absorbance of the monomer solutions and C=C concentration per ml.
The relationship (Figure 1) was used to determine the C=C molar concentration of each of the five composites from FTIR spectra data. The weight of methacrylate groups in each ml of composite was calculated by multiplying the molar concentration by the molecular weight of a methacrylate unit (69.081) (Rueggeberg, 1994; Bartoloni, 2000). The density of each composite was determined using a density determination kit (VPG214CN, Ohaus Corp., Pine Brook, NJ, USA) at room temperature. The weight percent of methacrylate groups (WPMG) in the uncured composite (WPMGu) was calculated by dividing the weight of the methacrylate groups in 1 ml composite in by its density (Rueggeberg, 1994; Bartoloni, 2000).
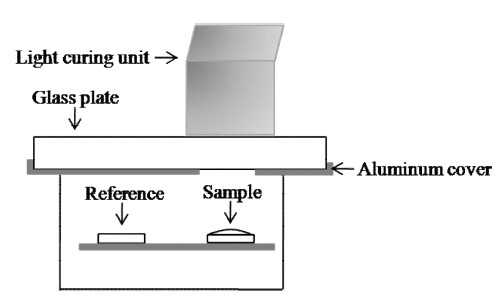
The number of moles of C=C per g of each composite was calculated by dividing the molar concentration by its density. The ΔHt was calculated by multiplying moles of the methacrylate groups in 1 g composite by theoretic heat release per mole reacted carbon double bond (56.92 kJ/mol) (Cadenaro et al., 2008). A differential scanning calorimeter (Shimadzu DSC-50, Kyoto, Japan) was used to measure the heat of polymerization as a function of the extent of polymerization of different composites. Two aluminum pans were placed in the sample holder of the calorimeter furnace: one contained the tested specimen (15 mg); and the other was empty (Suh et al., 2003) (Figure 2). The DSC chamber was covered by an aluminum lid with a round hole (6.8 mm diameter which is the same with diameter of aluminum pan) and a glass slide (25 × 25 × 1 mm) to allow light pass through and permit curing of the specimen inside the calorimeter. A glass plate (68 × 68 × 9 mm) was also used to further reduce the intensity so that the heat flow recorded by the DSC unit was within ± 40 mW. The light guide was positioned at a distance of 22 cm from the base of the sample chamber. The curing procedure was performed for up to 60 s at about ambient temperature in a nitrogenpurged environment. Calorimeter analysis consisted of two consecutive light exposures: the first exposure to the composite specimens to produce polymerization, and the second exposure to an empty aluminum pan to evaluate irradiation heat flow from the light-curing unit. The heat of reaction obtained from the first scan represented the sum of the exothermic effect due to monomer conversion plus the heat flow from the curing unit, while the heat flow measured in the second scan was attributed to the irradiation heat output of the lamp. The observed heat is based on the total area under the recorded curve. The heat of resin polymerization can be calculated by subtracting the heat value of the second exotherm from the value obtained after the first light exposure. The ΔHobs was calculated by dividing the heat of resin polymerization by its weight (15 mg). Degree of conversion (DC3) was determined as the percentage of ΔHt and ΔHobs as shown in Equation:
DC1 = ΔHobs/ ΔHt × 100%
where ΔHo is the heat of polymerization for 1 g of each composite and ΔHt is the theoretical amount of heat evolved during polymerization of 1 g composite.
The height of the absorbance peak of the aliphatic C=C group occurring around at 1636 cm-1 was determined for each cured composite specimen between two KBr plates. The cured specimens were obtained using the same curing method with internal standard. The thickness of each film was measured with a digital micrometer. The WPMG remaining in each cured film (WPMGc) was then determined using the optical constant established in following equation:
WPMGc = A/(T×K)
where WPMGc is weight percent of methacrylate groups in cured specimens; A is height of C=C absorbance peak height at 1636 cm-1; T is thickness of cured resin film (mm); and K is optical constant for methacrylate group (0.64) (Rueggeberg, 1994).
The DC of composite was then calculated by determining the property of WPMG available after curing, compared to the amount present prior to light exposure.
DC2 = [1 – WPMGc/WPMGU] × 100%
where DC2 is percent monomer conversion and WPMGU is weight percent methacrylate groups in uncured composite.
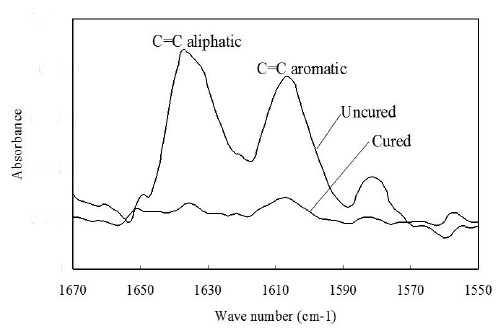
A sample of the uncured paste of each material was directly smeared onto the surface of ZnSe crystal. The absorbance peaks before curing were obtained from 10 scans at a resolution of 4 cm-1 on FTIR (Shimadzu Corp., Kyoto, Japan) with the MIRacle accessory (PIKE technologies Inc, Madison, WI, USA) from 1550 to 1670 cm-1. In order to produce the cured thin films, the paste was pressed between two glass slides with Mylar strips. A halogen light unit (Eliper Trilight, ESPE, MN, USA) was positioned with the curing tip located at 22 mm above the specimen. A thickness of 9 mm glass plate was used to contact with curing tip produce the same curing condition with DSC. Three specimen films of each composite were prepared. The degree of conversion (DC1) could be obtained from the ratio of the aliphatic C=C peak height to the aromatic C=C value before and after curing using conversional baseline techniques. The absorption ratio values for the cured specimens were obtained directly from cured thin resin films.
DC1 = [1 – C/U] × 100%
where C and U are the ratio of the aliphatic C=C peak height to aromatic C=C value of cured and uncured composites.
For each resin, the means obtained from the three different analysis methods were compared using a oneway analysis of variance (ANOVA) with Tukey’s post hoc test at a significance level of 0.05 (n=5 for each variable).
RESULTS
The linear regression analysis for the calibration curve (Figure 1) generated from various dilutions of MMA yielded an excellent fit (R2=0.997). It demonstrated that concentrations of unknown values of C=C could confidently be determined from their IR peak height.
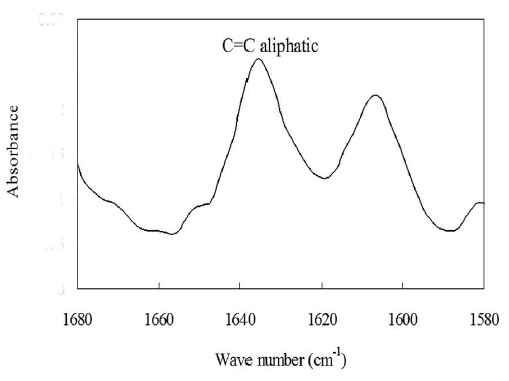
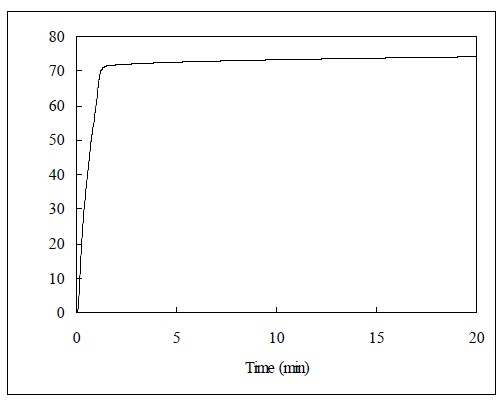
Figure 3 shows the IR spectrum of the uncured Clearfil AP-X. The peak height of aliphatic C=C was used to determine the C=C concentration from calibration curve. Figure 4 displays the IR spectra of Clearfil AP-X at the range of 1670-1550 cm-1 for internal standard method. The spectrum of cured specimen greatly decreased since the cured specimen can not contact with crystal completely, and thus decrease sensitivity. Figure 5 represents the polymerization exotherms of Clearfil AP-X during and after light exposure obtained from DSC.
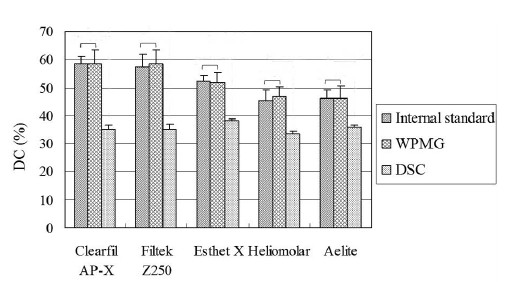
Figure 6 shows the DC values obtained from three methods and the results of statistical analysis. Internal standard and WPMG methods have no significant difference between each other for each composite (p>0.05). However, DSC method is significantly lower than the other two methods (p<0.05).
DISCUSSION
According to the statistical analysis, DC values of all materials used in this study obtained from WPMG method was similar to the results obtained from internal standard method. Correlated with previous results (Loshaek and Fox, 1953; Rueggeberg, 1994; Bartoloni, 2000), WPMG method is adaptable not only to liquid restorative materials (dentin bonding agents, resin adhesives or denture base materials) but also to highly filled composites. And the calibration curve also can be used to determine the concentration of C=C of composite, although it is obtained by liquid MMA. This investigation demonstrated that degree of conversion can be determined for commercial composites without functional groups acting as internal standards.
In this study, DC values obtained from DSC method are significantly lower than internal standard method (p<0.05). However, according to previous studies (Cadenaro et al., 2008; Schneider et al., 2008; Antonucci and Toth, 1983), DSC method is a useful method to determine DC. This may be mainly attributed to the fact that the concentration of uncured C=C was determined from calibration curve obtained from IR. According to Halvorson et al. (2003), most of the methacrylate functionality within the silicon layer is in a non-reactive environment because a highly condensed silane interphase limits mobility of the silane methacrylate, and hence its reactivity (Du and Zheng, 2008). While the C=C contained in silane methacrylate can be observed in IR spectrum. In other words, the mole concentrations of C=C obtained from calibration curve is higher than the real C=C from resins due to the effect of filler, which causes the theoretical exothermic heat to be higher. According to equation (Yoshida and Greener, 1993), the DC values may decrease due to the increasing values of denominator. Another explanation may be a basic difference in the measurements: FTIR spectroscopy measures only contain selected bonds but DSC measures all reactive groups that react liberating heat (Vijanen et al., 2007).
Although the WPMG method also used the calibration curve to calculate the concentrations of C=C in uncured materials, it did not significantly affect the DC results comparing with internal standard method. This may be attributed to the fact that the amounts of C=C contained in filler could be observed in both cured and uncured materials by IR. Therefore both WPMGU and WPMGc values contain the weight percentage of methacrylate in silane layer, not to cause significantly effect on DC values.
CONCLUSION
From the results obtained from this study, it can be concluded that calibration curve can be applied to WPMG method to calculate DC values and not for DSC method. The hypothesis tested in this study only partly accepted.
Acknowledgments
This research was supported by Kyungpook National University Research Fund, 2013.
References
-
JM Antonucci, EE Toth, Extent of polymerization of dental resins by differential scanning calorimetry, J Dent Res, (1983), 62, p121-25.
[https://doi.org/10.1177/00220345830620020701]

-
JA Bartoloni, DF Murchison, DT Wofford, NK Sarkar, Degree of conversion in denture base materials for varied polymerization techniques, J Oral Rehabil, (2000), 27, p488-493.
[https://doi.org/10.1046/j.1365-2842.2000.00536.x]

- SC Bayne, Dental biomaterials: where are we and where are we going?, J Dent Educ, (2005), 69, p571-585.
-
M Cadenaro, F Antoniolli, S Sauro, FR Tay, R Di Lenarda, C Prati et al, Degree of conversion and permeability of dental adhesives, Eur J Oral Sci, (2005), 113, p525-530.
[https://doi.org/10.1111/j.1600-0722.2005.00251.x]

-
M Cadenaro, L Breschi, F Antoniolli, CO Navarra, A Mazzoni, FR Tay et al, Degree of conversion of resin blends in relation to ethanol content and hydrophilicity, Dent Mater, (2008), 24, p1194-200.
[https://doi.org/10.1016/j.dental.2008.01.012]

-
MH Du, Y Zheng, Degree of conversion and mechanical properties studies of UDMA based meaterials for producing dental posts, Polym Compos, (2008), 29, p623-630.
[https://doi.org/10.1002/pc.20420]

-
JY Ha, SH Kim, KH Kim, TY Kwon, Influence of the volumes of bis-acryl and poly(methyl methacrylate) resins on their exothermic behavior during polymerization, Dent Mater J, (2011), 30, p336-342.
[https://doi.org/10.4012/dmj.2010-188]

-
RH Halvorson, RL Erickson, CL Davidson, The effect of filler and silane content on conversion of resin-based composite, Dent Mater, (2003), 19, p327-333.
[https://doi.org/10.1016/S0109-5641(02)00062-3]

-
S Imazato, JF McCabe, H Tarumi, A Ehara, S Ebisu, Degree of conversion of composites measured by DTA and FTIR, Dent Mater, (2001), 17, p178-183.
[https://doi.org/10.1016/S0109-5641(00)00066-X]

-
S Loshaek, TG Fox, Cross-linked polymers. І. Factors influencing the efficiency of cross-linking in copolymers of methyl methacrylate and glycol dimethacrylates, J Am Chem Soc, (1953), 75, p3544-3550.
[https://doi.org/10.1021/ja01110a068]

-
LG Moraes, RS Rocha, LM Menegazzo, EB de Araujo, K Yukimito, JC Moraes, Infrared spectroscopy: a tool for determination of the degree of conversion in dental composites, J Appl Oral Sci, (2008), 16, p145-149.
[https://doi.org/10.1590/S1678-77572008000200012]

-
FA Rueggeberg, Detemination of resin cure using infrared analysis without an internal standard, Dent Mater, (1994), 10, p282-286.
[https://doi.org/10.1016/0109-5641(94)90076-0]

-
LF Schneider, LM Cavalcante, S Consani, JL Ferracane, Effect of co-initiator ratio on the polymer properties of experimental resin composites formulated with camphorquinone and phenyl-propanedione, Dent Mater, (2008), 25, p369-375.
[https://doi.org/10.1016/j.dental.2008.08.003]

- BI Suh, L Feng, DH Pashley, FR Tay, Factors contributing to the incompatibility between simplified-step adhesives and chemically-cured or dualcured composites. Part III. Effect of acidic resin monomers, J Adhes Dent, (2003), 5, p267-282.
-
EK Viljanen, M Skrifvars, PK Vallittu, Dendritic copolymers and particulate filler composites for dental applications: degree of conversion and thermal properties, Dent Mater, (2007), 23, p1420-1427.
[https://doi.org/10.1016/j.dental.2006.11.028]

-
K Yoshida, EH Greener, Effects of two amine reducing agents on the degree of conversion and physical properties of an unfilled light-cured resin, Dent Mater, (1993), 9, p246-251.
[https://doi.org/10.1016/0109-5641(93)90069-3]