Synthesis and in vitro evaluation of silica coated gold nanoparticles for hyperthermia
본 연구는 암세포를 효과적으로 사멸시키기 위하여 실리카가 코팅된 골드나노입자에 의한 근적외선 조사로 온열 효과를 보고자 하였다. 골드 나노 입자를 생성한 후 코어-쉘 구조를 확인하기 위하여 TEM으로 측정하였으며, 흡광도 변화를 측정하기 위하여 UV 를 사용하였다. 제작된 시편의 생물학적 독성평가를 하기 위하여 일반세포(L 929 cell)와 암세포(HeLa cell)로 MTT assay를 시행하였다. 일반세포와 암세포는 생존률이 80 % 이상으로, 독성이 없는 것으로 확인되었다. 온열 치료를 유도하기 위하여 근적외선 조사를 시행한 결과, 조사후의 온도가 40 ℃ 이상이 되었을 때 일반세포에서는 30 % 미만 사멸한 반면 암세포에서는 70 % 이상 사멸하였다.
Keywords:
core-shell nanoparticles, gold nanoparticles, hyperthermia, silica shellINTRODUCTION
Heating of organs and tissues in cancer treatment was first reported in 1968. The report described the heating of the tissue and the destruction of cancer by thermal therapy and chemotherapy with a later emphasis on radiation therapy.1 Gold nanoparticles (also known as gold colloids) of 2 nm to more than 100 nm in diameter can be synthesized by the controlled reduction of an aqueous HAuCl4 solution using different agents under a range of conditions. The most commonly used reduction agent is citrate, which can reduce gold nanoparticles to an almost monodispersed form.2-13 Murrary et al. developed a three-step procedure involving the use of an amine-terminated silane coupling agent as the primer to allow for subsequent silica adhesion. Hall et al. extended the indirect procedure to synthesize silica coated gold nanoparticles using the base-catalyzed co-condensation of tetraethyl orthosilicate (TEOS) and organoethoxysilane precursors.14 The silica-shell thickness, refractive index of the solvent and optical spectra of silica coated gold colloids are in good agreement with the predictions made by Mie theory.15 The results from these researchers indicated that silica-coated gold nanoparticles tend to aggregate when kept for a long time after coating with silane coupling agents. Optical imaging, including those that employ gold nanoparticles as the contrast agents, has very limited applications in human studies. The absorbance of all biomolecules reaches a minimum value in the near-infrared region (NIR; 700~900 nm), which provides a relatively clear window for optical imaging. In addition, the wavelength of absorbance shifts slightly when the silica shell is thin. The reason why the wavelength is so important is because the human body is composed mainly of water. Therefore, those with a short-wavelength will have a low level of penetration through the skin whereas those with a long-wavelength (those in NIR area) will have deeper penetration and could be used internally. Based on previous researches, this study coated silica on gold nanoparticles using the direct method of polymerization without silane coupling agents. The aim of this study was to synthesize and evaluate silica-coated gold nanoparticles that could lead to thermotherapy in cancer treatment.
MATERIALS AND METHODS
Synthesis of gold and silica coated gold nanoparticles
A gold nanoparticle colloid was prepared using sodium citrate reduction. Approximately 100 mL of 0.01% (w/v) HAuCl4 (Sigma) was heated to boiling point, followed by the addition of 10 mL of a 1% (w/v) sodium citrate solution under high-speed stirring. The resulting mixture was heated and stirred for 30 min. Silica coated gold nanoparticles were divided into two groups according to the TEOS concentration (Sigma): (1) 5 mM of TEOS, (2) 10 mM of TEOS.
The silica shell could be coated directly onto the gold surface employing a slightly modified procedure using a silane coupling agent. 4 mL of the gold nanoparticle suspension was added to 20 mL ethanol. Under continuous magnetic stirring, 0.5 mL of an ammonia solution and two TEOS concentrations (5 mM, 10 mM) were consecutively added to the reaction mixture to obtain a different silica shell thickness. Consequently, three types of particles were named gold nanoparticles (GN), silica coated gold nanoparticles 5 mM of TEOS (5SGN), and silica coated gold nanoparticles 10 mM of TEOS (10SGN).
Characterization techniques
The mean size and morphology of the particles were estimated by transmission electron microscopy. The size distribution of the determined GN, 5SGN, and 10SGN were measured by dynamic light scattering to confirm the increasing silica-shell thicknesses. The change in the absorbance of GN, 5SGN, and 10SGN in the colloids were observed by UV-visible spectroscopy.
Measuring the heat generation
Silica coated gold nanoparticles 5SGN and 10SGN, 1, 2, 3, 4, and 5 g in weight, were dissolved separately in 3 mL of distilled water to produce 0.03, 0.07, 0.1, 0.13, and 0.17 wt% colloids, respectively. The samples were immersed on a 12 well plate for each concentration, and irradiated with a NIR lamp for 10 min. The changes in temperature after NIR lamp irradiation were measured using electronic thermometers (start temperature: 36°C).
Cytotoxicity studies
In this study, we were used normal cell line in L929 and cancer cell line in Hela. Both L929 and carcinoma cells (HeLa) cells were grown in RPMI1640 and DMEM medium with 10% fetal bovine serum and 1% penicillin at 37°C in 5% CO2. Each cell line was seeded into 96-well plates at 5 × 104 cells/mL. An evaluation of the effect of 5SGN and 10SGN on the cell viability was determined using an 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay (ISO10993-5).
The necrosis of carcinoma cells by 5SGN and 10SGN was measured using a MTT assay, after 10 min irradiation with NIR. The experimental group was divided into 4 groups. The 1st, 2nd, 3rd and 4th group was the control with the medium only, GN, 5SGN and 10SGN, respectively.
RESULTS AND DISCUSSION
Characterization of silica coated gold nanoparticles
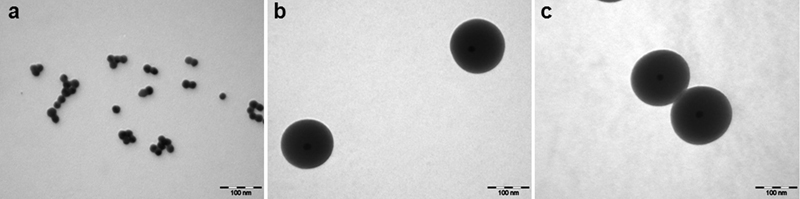
Before coating, the diameter of the GN was 25 nm based on transmission electron microscopy (TEM) (Fig. 1A, 1B and 1C) show typical TEM images of the 5SGN and 10SGN colloids with different silica-shell thicknesses. The core-shell structure was evident in TEM Photograph Fig. 1B and 1C shows 5SGN and 10SGN with a value of 120 nm and 160 nm, respectively. The silica shell thickness increased with increasing TEOS concentration.
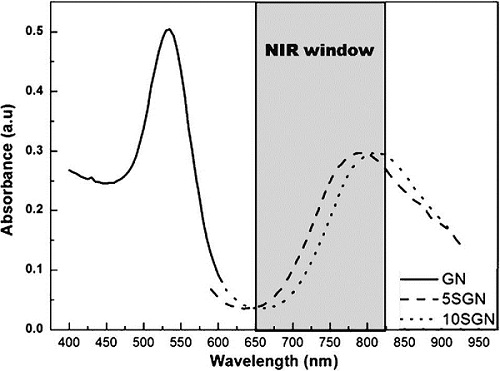
Dynamic light scattering (DLS) showed that GN, 5SGN and 10SGN had a thickness of 25.05 nm, 119.18 and 162.25 nm, respectively, which are similar to the TEM results. This means that the silica shell thickness increased with increasing TEOS concentration. Figure 2 shows the UV-visible absorbance spectra of GN, 5SGN and 10SGN. The maximum absorbance wavelength of GN was 542.8 nm and those of 5SGN and 10SGN were 758.2 nm and 799.5 nm, respectively. A short wavelength with 540 nm was absorbed very weakly by the skin. On the other hand, a wavelength >700 nm had a much higher absorption rate and could be used in the human body.
Encapsulation of individually separated nanoparticles with silica-based shells is advantageous for bioconjugation and application to (nano) biotechnology, because silica is non-toxic and easy to functionalize, and protects core nanoparticles from deleterious reactions such as oxidation. As a core, gold nanoparticles show a good biocompatibility and have potential application to therapeutic areas. In particular, the absorption of anisotropically shaped gold nanoparticles or nanoshells in the near-infrared (NIR) region makes photo-thermal therapy possible and therefore much effort is currently being made for applications of gold-based nanostructures to cancer hyperthermia.16 In this study, as shown in Figure 2, silica coated gold nanoparticle has been shown in the NIR region. Encapsulation of gold nanoparticles has been shown in the NIR region. Encapsulation of gold nanoparticles with a silica shell would be a simple method for stabilizing gold nanoparticles and making them applicable to (nano) biotechnological areas.
Silica encapsulation of individually separated nanoparticles is advantageous for bioconjugation and application to (nano) biotechnology, because silica is nontoxic and easy to functionalize, and protects core nanoparticles from deleterious reactions such as oxidation. Especially in the case of gold nanoparticles silica encapsulation would be one of the methods for stabilizing gold nanoparticles and making them applicable to (nano) biochnological areas 16.
Heat generation
The heat generated by nanoparticles can be used to melt the nanoparticle themselves and modify their shape or to affect the surrounding materials, such as to destroy organic molecules1617 or to trigger chemical reactions. Both theoretical calculations and experimental measurements have been conducted to better understand the heating process of nanoparticles and the effects on the surrounding medium. Hyperthermia-based therapy is a form of cancer treatment that uses an elevated temperature to kill the tumor tissue. Compared to the more conventional surgical procedures, hyperthermia is a less invasive approach that could be used for small, non-defined or embedded tumor where surgical resection is not feasible.
The NIR region of the spectrum provides maximal penetration of light through the body due to relatively lower scattering and adsorption from the intrinsic tissue chromophores. The heat generation properties and in vitro cellular uptake of the particles are aspects under investigation.
Table I lists the change in temperature of SGN (silica coated gold nanoparticles). The temperature at concentrations of 0.13 and 0.17 mg/mL could affect normal cells. When the NIR lamp was irradiated for 10 min, a concentration of 0.1 mg/mL was measured as the optimal concentration for the destruction of temperature sensitive cancer cells.
In vitro cytotoxicity
Nanoparticle aggregation is a hurdle that needs to be overcome before nanoparticles can be applied in vivo, cluster of nanoparticles may not be filtered and excreted in living organisms, leading to chronic toxicity and other negative side effects in biodistribution. In order to reduce aggregation of nanoparticles, one solution has been to include a final incubation period in 3-hydroxysilylpropyl methylphosphonate during the synthesis process of fluorescent mesoporous silica surface was modified with inert and hydrophilic phosphonate group to prevent aggregation caused by the interparticle hydrogen bonding interaction between the anionic silanol groups and the unreacted cationic amine group.18
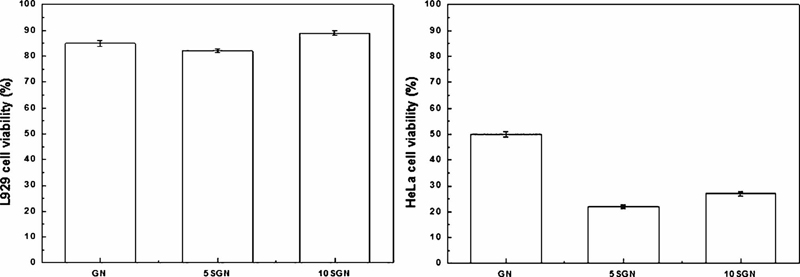
In this study, the L929 cell and HeLa cell viability after exposure to GN, 5SGN, and 10SGN were >80%; they could be considered to be non-cytotoxic. The necrosis of carcinoma cells using silica-coated gold core-shell nanoparticles was also measured. Each cell was exposed to GN, 5SGN and 10SGN and irradiated with a NIR lamp.
The cell necrosis effect of the prepared nanoparticles after exposure to the NIR lamp was examined using a MTT assay (Figure 3). 5SGN and 10SGN showed 82.7% and 89.4% viability of L929 cells but 22.7% and 26.4% viability of HeLa cells after exposure for 10 min. As a result, 5SGN and 10SGN are considered to be more effective on cancer cell necrosis than GN.
This study synthesized and evaluated SGN for inducing hyperthermia in cancer treatment. GN could be coated directly with a uniform silica shell. The coating thickness could be controlled from tens to hundred nanometers by selecting the appropriate TEOS concentration. SGN is expected to be a useful hyperthermic cancer-treatment because it exhibited effective heat generation under near infrared as well as tumor cell necrosis in vitro.
Acknowledgments
This study was supported by Brain Korea 21 PLUS Project, Yonsei University, College of Dentistry
References
-
GG Harrison, SJ Saunders, JF Biebuyck, R Hickman, DM Dent, V Weaver, J Terblanche, Anaesthetic-induced malignant hyperpyrexia and a method for its prediction, Br J Anaesth, (1969), 41, p844-855.
[https://doi.org/10.1093/bja/41.10.844]

-
D Li, Q He, Y Cui, L Duan, J Li, Immobilization of glucose oxidase onto gold nanoparticles with enhanced thermostability, Biochem Biophys Res Commun, (2007), 355, p488-493.
[https://doi.org/10.1016/j.bbrc.2007.01.183]

-
S-M Ryou, J-M Kim, J-H Yeom, S Hyun, S Kim, MS Han, SW Kim, J Bae, S Rhee, K Lee, Gold nanoparticle-assisted delivery of small, highly structured RNA into the nuclei of human cells, Biochem Biophys Res Commun, (2011), 416, p178-183.
[https://doi.org/10.1016/j.bbrc.2011.11.020]

-
J Turkevich, PC Stevenson, J Hillier, A study of the nucleation and growth processes in the synthesis of colloidal gold, Discuss Faraday Soc, (1951), 11, p55-75.
[https://doi.org/10.1039/df9511100055]

-
X Huang, PK Jain, IH El-Sayed, MA El-Sayed, Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy, Nanomedicine, (2007), 2, p681-693.
[https://doi.org/10.2217/17435889.2.5.681]

-
WB Tan, Y Zhang, Surface modification of gold and quantum dot nanoparticles with chitosan for bioapplications, Journal of Biomedical Materials Research Part A, (2005), 75, p56-62.
[https://doi.org/10.1002/jbm.a.30410]

-
RD Ross, RK Roeder, Binding affinity of surface functionalized gold nanoparticles to hydroxyapatite, Journal of Biomedical Materials Research Part A, (2011), 99, p58-66.
[https://doi.org/10.1002/jbm.a.33165]

-
MJ Cozad, SL Bachman, SA Grant, Assessment of decellularized porcine diaphragm conjugated with gold nanomaterials as a tissue scaffold for wound healing, Journal of Biomedical Materials Research Part A, (2011), 99, p426-434.
[https://doi.org/10.1002/jbm.a.33182]

-
Z Liang, Y Liu, X Li, Q Wu, J Yu, S Luo, L Lai, S Liu, Surface modified gold nanoshells for enhanced cellular uptake, Journal of Biomedical Materials Research Part A, (2011), 98, p479-487.
[https://doi.org/10.1002/jbm.a.33068]

-
Y Lee, KE Geckeler, Cytotoxicity and cellular uptake of lysozyme stabilized gold nanoparticles, Journal of Biomedical Materials Research Part A, (2012), 100, p848-855.
[https://doi.org/10.1002/jbm.a.34020]

- CW Chou, Sh Hsu, PH Wang, Biostability and biocompatibility of poly (ether) urethane containing gold or silver nanoparticles in a porcine model, Journal of Biomedical Materials Research Part A, (2008), 84, p785-794.
-
TH Kim, M Kim, HS Park, US Shin, MS Gong, HW Kim, Size-dependent cellular toxicity of silver nanoparticles, Journal of Biomedical Materials Research Part A, (2012), 100, p1033-1043.
[https://doi.org/10.1002/jbm.a.34053]

-
DE Owens, JK Eby, Y Jian, NA Peppas, Temperature-responsive polymer-gold nanocomposites as intelligent therapeutic systems, Journal of Biomedical Materials Research Part A, (2007), 83, p692-695.
[https://doi.org/10.1002/jbm.a.31284]

- R Simon, SA Davis, S Mann, Cocondensation of organosilica hybrid shells on nanoparticle templates: a direct synthetic route to functionalized core-shell colloids, Langmuir, (2000), 16, p1454-1456.
-
LM Liz-Marzan, M Giersig, P Mulvaney, Synthesis of nanosized gold-silica core-shell particles, Langmuir, (1996), 12, p4329-4335.
[https://doi.org/10.1021/la9601871]

- A Lin, L Hirsch, M-H Lee, J Barton, N Halas, J West, R Drezek, Nanoshell-enabled photonics-based imaging and therapy of cancer, Technol Cancer Res Treat, (2004), 3, p33-40.
-
CM Pitsillides, EK Joe, X Wei, R Anderson, CP Lin, Selective cell targeting with light-absorbing microparticles and nanoparticles, Biophys J, (2003), 84, p4023-4032.
[https://doi.org/10.1016/S0006-3495(03)75128-5]

-
C Hom, J Lu, F Tamanoi, Silica nanoparticles as a delivery system for nucleic acidbased reagents, J Mater Chem, (2009), 19, p6308-6316.
[https://doi.org/10.1039/b904197d]