In Vitro Effectiveness of Remineralizing Treatments for Protecting Early Carious Lesions from Further Acid Dissolution
본 연구에서는 재광화 재제로 개발된 casein phosphopeptide stabilized amorphous calcium phosphate(CPP-ACP)와 베타 삼인산칼슘(fTCP)으로 처리한 법랑질 병소가 재탈회 되었을 때 추가적인 산의 침투에 대한 병소의 저항성을 평가하고자 사람의 소구치를 이용하여 법랑질 시편을 제작한 후, 탈회용액에 넣어 법랑질 병소를 만들고, 재광화 제재를 도포하여 인공타액에 보관한 후 추가적인 탈회를 실시하였다. 시편을 표면에 수직으로 잘라 형광색소로 염색하고 공초점 레이저 주사현미경을 이용하여 재광화 처리 후 및 2차 탈회 후의 병소의 깊이를 측정하였고, 주사전자현미경을 이용하여 병소의 표면형상의 변화를 관찰하였다. 2차 탈회 후 fTCP 처리군에서는 재광화 후와 비교하여 병소의 깊이가 증가하지 않았고, 재광화 처리를 하지 않은 대조군과 CPP-ACP 처리군에서는 병소의 깊이가 증가하였으나 CPP-ACP처리군에서 깊이의 증가량이 유의하게 작았다. 재광화 처리군의 표면에 형성된 무기 침전물은 fTCP 처리군에서는 2차 탈회 후에도 잘 유지된 반면, CPP-ACP처리군에서는 2차 탈회 후 부분적으로 탈락되어 산의 침투에 대한 적절한 방어벽을 형성하지 못한 것으로 보였다.
Keywords:
Enamel lesions, Further acid attack, Lesion depth, Remineralizing treatments, Surface configuration서 론
Dental caries is a dynamic process and demineralization can be reversed if the pH is neutralized and there are sufficient calcium and phosphate ions available in the immediate environment (Reynolds, 1997). The non-invasive treatment of early carious lesions by remineralization with an increased resistance to further acid challenge has the potential to be a major advance in the clinical management of the disease. To date, fluoride therapy has remained the most popular of all caries prevention and fluoride is considered to be the gold standard active agent for remineralization (Geiger et al., 1992). However, since fluoride cannot be effective in promoting remineralization without calcium, many remineralization agents have been designed to potentiate fluoride by elevating oral calcium concentrations, either directly, by delivery into enamel lesions, indirectly, by elevating concentrations in plaque and saliva, or both (Lynch and Smith, 2012). Recently, novel calcium phosphate-based remineralization delivery systems have been developed for clinical application.
Anticariogenic mechanism of casein phosphopeptide stabilized amorphous calcium phosphate (CPP-ACP) involves the incorporation of nanocomplexes into dental plaque and onto the tooth surface (Rose, 2000). It has been claimed that CPP acts as a delivery vehicle to colocalize calcium and phosphate ions, which results in increasing the level of the ions at the tooth surface (Reynolds et al., 2008) and supragingival plaque (Reynolds et al., 2003). The ions are freely bioavailable to diffuse down into enamel subsurface lesions by concentration gradients, thereby effectively depressing demineralization and enhancing remineralization of the lesions in animal and human caries models (Reynolds et al., 1995; Reynolds, 1997; Cai et al., 2003). Addition of fluoride (0.2% NaF) to CPP-ACP, resultant CPP-ACPF showed marginally more amount of remineralization in enamel subsurface lesion than CPP-ACP only because of the added benefit of fluoride (Jayarajan et al., 2011).
Beta-tricalcium phosphate (β-TCP) is a known precursor to hydroxyapatite (HA) formation because it appears as a transitional phase in HA conversion (Amiad, 1998). It has recently been introduced to dental products and manufacturers claimed that it is able to remineralize white spot lesions. Since calcium fluoride (CaF2) can be rapidly formed when calcium phosphate and fluoride are introduced together in a dental material due to the high reactivity of bioavailable fluoride and calcium (Kawska et al., 2006), β-TCP was modified with ionic surfactant, sodium lauryl sulfate (SLS) using mechanochemical ball milling process (Karlinsey and Mackey, 2009; Karlinsey et al., 2010) to produce functionalized β-TCP (fTCP). There have been several reports on the benefits observed through a combination of low level of fluoride and fTCP to produce greater mineral recovery and reduce the lesion depth by building stronger, more acidresistant mineral relative to fluoride alone (Karlinsey et al., 2010; Mathews et al., 2012; Mensinkai et al., 2012). However, despite the numerous studies have shown that calcium phosphate-based remineralization regimen can reverse the demineralized early carious lesions, it remains unknown whether the remineralized incipient lesions would be still resistant to further acid attack.
Therefore, the aim of the present study was to evaluate the ability of topically applied remineralizing agents in inhibiting the progression of artificial enamel lesions treated with commercially available remineralizing agents and further exposed to a deminerlaizing solution in vitro. Confocal laser scanning microscopy (CLSM) was utilized to measure the mineral change of the lesions because it allows an accurate quantitative analysis of surface and subsurface areas, providing estimation of lesion depth (Gonzalez-Cabezas et al., 1998; Behnan et al., 2010).
MATERIALS AND METHODS
1. Preparation of an artificially demineralized specimen
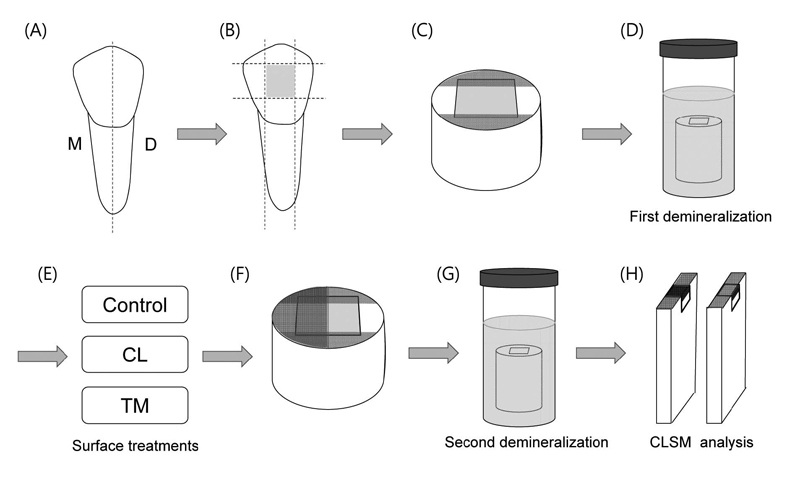
Twenty four human premolars with no caries and restorations which extracted for orthodontic reasons were collected with informed signed consent obtained under a protocol approved by the Ethics Committee of School of Dentistry, Kyungpook National University. The teeth were cleaned, disinfected in 0.5% chloramine T, and stored in distilled water at 4°C until use. Each tooth was sectioned longitudinally from buccal to lingual into two halves to give a total of 48 enamel slabs (approximately 3×4×3 mm3) using a low-speed diamond saw (Isomet, Buehler Ltd., Lake Bluff, IL, USA). The specimens were embedded in a 10 mm diameter cylindrical silicone mold using an orthodontic resin (Dentsply Caulk, Milford, DE, USA) and the surfaces were wet polished in an automatic polisher (Metaserv 250 Grinder-Polisher, Buehler Ltd.) with 600- and 1200-grit SiC papers, followed by a final polishing using 3000- and 4000-grit lapping film (3M ESPE, St. Paul, MN, USA) (Paris et al., 2006). The specimens were then covered with acid-resistant nail varnish leaving a window (2×3 mm2) of uncovered enamel to make a reference point for measuring lesion depth. The schematic illustration of the test procedure is shown in Figure 1.
To create artificial enamel lesions (approximately 100- 150 μm in depth) in the uncovered areas, the specimens were immersed in 5 mL of demineralizing solution containing 2 mM CaCl2·2H2O, 2 mM KH2PO4 and 75 mM acetate buffer (Sigma-Aldrich, St. Louis, MO, USA) at pH 4.3 for 14 d at 37°C (Trairatvorakul et al., 2008). The solutions were changed every other day.
2. Application of remineralizing agents
After demineralization, the specimens were randomly divided into three groups according to surface treatments: 1) Control: no treatment; 2) CL: remineralization with fTCP (ClinproTM Tooth Crème, 3M ESPE); 3) TM: remineralization with CPP-ACPF (Tooth Mousse Plus, GC, Tokyo, Japan).
Test materials used in this study and application procedures are listed in Table 1. The application time of each material was decided according to the manufacturer's recommendations. For group CL, specimens were rinsed with deionized (DI) water for 15 s and ClinproTM Tooth Crème was applied on the surface of the specimens with a microbrush. The specimens were then left undisturbed for 2 min. After the treatment, remaining paste was removed with cotton balls and the specimens were immersed in 5 mL of artificial saliva solution, which containing 5.36 mM KCl, 6.84 mM NaCl, 6.16 mM CaCl2·2H2O, 4.42 mM (NaH2PO4) · 2H2O, 0.02 mM (Na2S) ·9H2O, and 16.06 mM urea (Sigma-Aldrich) at room temperature, neutral pH (Liu et al., 2011). The artificial saliva solutions were changed daily after the last treatment of the day. The test material was applied on the enamel surface three times a day for 10 d to simulate the normal recommended daily oral prophylaxis. Specimens in group TM were treated with Tooth Mousse Plus, in the same way as for group CL, but with 3-min application time. For the control group, specimens were stored in the artificial saliva for 10 d at 37°C without any treatment after the lesion formation.
3. Second demineralization
With the exception of the specimens for scanning electron microscopy (SEM), one half of the treated surface of the specimens was covered with nail varnish perpendicularly to the previously vanished lines, in order to compare the lesion depth before and after the second demineralization (Figure 1F). Subsequently, the specimens were reexposed to the demineralizing solution for 14 d at 37˚C (Paris et al., 2006). The solutions were changed every other day.
4. Scanning electron microscopy (SEM)
For SEM analysis of cross-sectioned surfaces of the specimens, two specimens per group were cut vertically to their surfaces and dehydrated in ascending grades of ethanol: 25% for 20 min, 50% for 20 min, 75% for 20 min, 95% for 30 min, and 100% for 60 min. Prior to SEM examination, the specimens were dried by immersing in hexamethyldisilazane (Sigma-Aldrich) for 15 min, then placed on a filter paper inside a covered glass vial for at least 24 h. The FE-SEM observation with a S-4300 (Hitachi, Tokyo, Japan) was made after platinum/gold sputtering on the samples at 15 kV.
5. Confocal laser scanning microscopy (CLSM)
In order to evaluate the progression of the artificial caries lesion after the application of the remineralizing agents, the specimens were cut perpendicularly to the surface using a low-speed diamond saw (Isomet, Buehler Ltd.), providing two parts, one for after remineralization and the other for after second demineralization (Figure 1H). For staining of the demineralized areas, the specimens were immersed in 0.1 mM rhodamine B solution (Sigma-Aldrich) for 1 h. The specimens were then thoroughly washed in DI water and observed using a confocal laser scanning microscope (LSM 700, Carl Zeiss, Oberkochen, Germany) in fluorescence mode at 50× magnification. The excitation light had a maximum wavelength at 555 nm. Images were recorded with a size of 1279.10 × 1279.10 μm2 and a lateral resolution of 1024 × 1024 pixels.
The images were analyzed using ImageJ software (NIH, Rockville Pike, MD, USA). Lesion depth was defined as the distance from the reference on the surface of the specimen to the point where the red value clearly changed to grey one, measured at three points per image, and the mean value was calculated. For each specimen, lesion depths were measured on the exposed surface (after the second demineralization) as well as on the varnished surface (after remineralization).
6. Statistical analysis
Lesion depths after the remineralization and the second demineralization among groups were analyzed with one-way ANOVA and Tukey's tests. A paired t-test was also used to evaluate the lesion progression after the second demineralization within the group. All statistical analyses were performed using the SPSS 18.0 (SPSS Inc. Chicago, IL, USA) at a significance level of 0.05.
RESULTS
The representative SEM micrographs showing the changes in the surface configuration of the untreated and treated enamel lesions before and after the second demineralization are presented in Figure 2. The control group in which no surface treatment was done showed further demineralization and irregular pit formation after the second demineralization (Figure 2B and 2C) compared to the relatively smooth demineralized surface after the first demineralization (Figure 2A). When the demnineralized enamel surfaces were treated with the remineralizing agents, it could be clearly seen that the surfaces were covered with an amorphous deposit, which completely obscured the underlying prism structure (Figure 2D and 2G). Following the second demineralization, the homogeneous mineral coating on the surface was still existed in group CL (Figure 2E), while the demineralized surface was partly exposed due to the detachment of the coating in group TM (Figure 2H). For group CL, the cross-sectional surface showed that there was no gap between the underlying enamel surface and the mineral sediment (Figure 2F). Although the specimens in group TM also exhibited the amorphous deposit over the demineralized surface (Figure 2G), the surface deposit was separated from the enamel surface after the second demineralization, forming the gap (Figure 2H and Figure 2I).
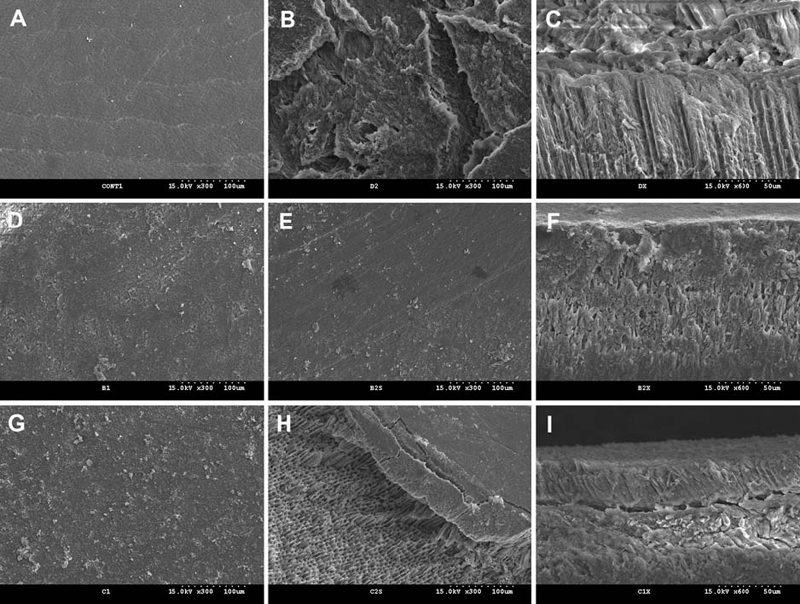
Representative scanning electron micrographs showing the changes in the surface configuration of the enamel lesions before and after the second demineralization. (A) the demineralized surface after the lesion formation in the control (300×); (B) the enamel surface after the second demineralization in the control (300×); (C) the cross-sectional view of (B) (600×); (D) the enamel surface treated with CL (300×); (E) the enamel surface of (D) after the second demineralization in CL (300×); (F) The cross-sectional view of (E) (600×); (G) the enamel surface treated with TM (300×); (H) the enamel surface of (G) after the second demineralization (300×); (I) The cross-sectional view of (H) (600×).
As for CLSM images, porous regions of the specimens appeared red when compared with dark normal enamel or sealed pores, indicating that they were stained with fluorescent rhodamine B dye. The outermost layer of the lesion was removed and cavity was created in the specimens of all groups during the formation of the artificial lesion and the subsequent surface treatment (Figures 3A1, 3B1, and 3C1). After the remineralization in groups CL and TM, the lesion was covered with the surface deposit and lesion depth was lower than the control even though group TM presented inhomogeneous surface layer with porous defects within it (Figure 3B1 and 3C1). A hypermineralized zone underneath the mineral coating appeared darker than normal enamel in the specimens of group CL. On the contrary, for group TM, there was no evidence of hypermineralization and intensely stained demineralized layer was observed under the surface deposit. After the second demineralization, a remarkable lesion progression was evident in the untreated control (Figure 3A2). The lesion applied with CL did not progress and the hypermineralized zone still remained on the surface, although the thickness of the amorphous layer was decreased (Figure 3B2). For the specimens treated with TM, substantial loss of the surface deposit was evident and the lesion depth was increased (Figure 3C2).
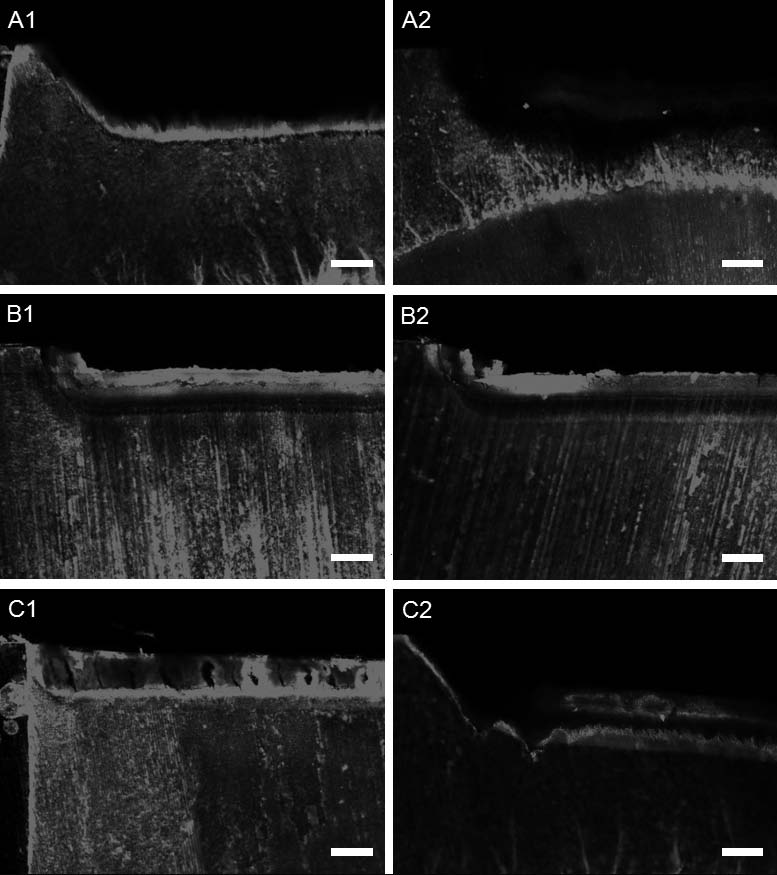
CLSM images of the untreated and the treated enamel lesions before and after the second demineralization (50×). (A1) the demineralized lesion in the control; (A2) the remarkable lesion progression after the second demineralization in the control; (B1) the lesion applied with CL; (B2) the enamel lesion of (B1) after the second demineralization; (C1) the lesion applied with TM; (C2) the enamel lesion of (C1) after the second demineralization (Scale bar = 100 μm).
The results of lesion depth according to the CLSM images of the tooth surface are shown in Table 2. To determine the lesion depth, the border between the lesion body and the advancing lesion front was defined through the extent of the labeling with the fluorescent dye (Pearce and Nelson, 1989). The control group produced the largest mean lesion area both before and after the second remineralization ranging between 212.26 μm and 327.32 μm (p<0.001). When the remineralizing agents were applied on the surface of the lesion, the lesion depths were significantly lower than the control group (p<0.05), and the lesion of group CL was significantly smaller than that of group TM (p<0.05). Similarly, after the second demineralization, the lowest lesion depth was obtained with group CL, followed by TM and the control. When lesion depths were compared before and after the second demineralization for the same treatments, there was no significant lesion progression in group CL (p>0.05), whereas group TM and the control showed a significant increase in the depth (p<0.001).
DISCUSSION
In the present study, the remineralizing agents based on CPP-ACPF and fTCP was used to evaluate the effectiveness of remineralizing treatments for protecting early carious lesions from further acid challenge. fTCP revealed similar lesion depths before and after the second demineralization, implying that fTCP may be able to inhibit further lesion progression. In addition, it was found that fTCP produced more stable mineral deposit that had a lower solubility and was more acidresistant compared to CPP-ACPF. Although CPP-ACPF also exhibited surface precipitation on the lesion, it was defective and vulnerable to the acid attack.
As regards remineralization of the demineralized lesion, the lesion treated with CPP-ACPF or fTCP was shallower than that of the untreated control immersed in artificial saliva. The lower lesion depth can be accounted by the success of the remineralizing therapy. As shown in the CLSM images, the hypermineralized zone underneath the surface deposit appeared darker than the normal enamel in the specimen of fTCP. The dark zone seems to be remineralized enamel lesion. On the contrary, CPP-ACPF does not appear to be as effective as fTCP in remineralization potential because there was no evidence of the remineralized zone in the lesion and the lesion depth was higher than in fTCP even after the remineralizing treatment. However, in this study, the lesion depth on baseline after the first demineralization was not measured since a previous study reported that there was no significant difference in the depth of artificially induced enamel lesions among experimental groups (Paris et al., 2006). Moreover, with CLSM analysis, tooth sections for baseline measurement could not be used again for the subsequent measurements such as after the remineralization and the second demineralization. Baseline values could offer a better comparison for the remineralization capability of the treatments.
The naturally occurring early enamel lesions are non-cavitated and have intact surface layer. Ideally, a remineralization system should supply stabilized bioavailable calcium, phosphate, and fluoride ions that favor subsurface mineral gain than deposition only in the surface layer (Cochrane et al., 2010). Salivary remineralization of enamel promoted by topical fluoride, particularly in high concentration, has been shown to give rise to predominantly surface remineralization (Willmot, 2004). Surface-only remineralization does little to improve the aesthetics and structural properties of the deeper lesion. In term of the lesion progression after the further acid attack in this study, the lesion area was smaller in the treatment groups than in the control. For both CPP-ACPF and fTCP specimens, the mineral sediment entirely covered the previously created lesions and acted as a physical barrier from further dissolution, suggesting fluoroapatite (FA) formation on the surface. However, unlike fTCP specimens, remineralization by the mineral gain in the lesion body was not obtained and further lesion progression was observed in CPP-ACPF specimens. This finding is in accordance with the results of the previous study, in which there was no increase in cross-sectional microhardness at the enamel subsurface and CPPACPF failed to remineralize the subsurface level in depth (Lata et al., 2010). The reason could be that fluoride ions and CPP-ACP were not able to penetrate the subsurface enamel area due to surface zone mineralization (ten Cate and Arends, 1980; ten Cate, 1990). Rapid deposition of FA forms a firm surface layer, which is more resistance to further demineralization. However, at the same time, it is resistance to penetration of calcium and phosphate ions required to rebuild the lesion in depth. Also, delivery of a new agent (i.e., calcium ions) and fluoride simultaneously from single-phase products may present formulation challenges, such as long-term fluoride compatibility. Although calcium-containing technologies aimed at providing anti-caries benefits, addition of these ingredients to a fluoride-containing product may result in significant decreases in fluoride’s well-established performance over the product’s shelf-life (Pfarrer and Karlinsey, 2009).
In contrast, fTCP induced enamel remineralization and inhibited further lesion progression despite presence of the surface precipitation, which implies calciumbased agent has actually reached the target tissue and resulted in anti-caries benefits (Sullivan et al., 2001). Sodium lauryl sulfate (SLS) is a synthetic anionic detergent widely used in dental formulations (Barkvoll et al., 1988b; Rodriguez-Hornedo and Murphy, 2004) owing to its high affinity for hydroxyapatite (HA) powder and enamel (Barkvoll et al., 1988a). β-TCP milled with SLS, this proprietary formula was reported to successfully integrate components, enhancing, rather than compromising the products's performance and minimize unwanted interaction among ingredients (White, 1995). Because a protective barrier is created around the calcium allowing it to coexist with the fluoride ions during the manufacturing process, fTCP may be incorporated into topically applied fluoride-containing preparations without negatively affecting the proven benefits of fluoride (Karlinsey and Mackey, 2009; Pfarrer and Karlinsey, 2009). Therefore, it is suggested that diffusion of bioavailable ions into the lesion body as well as the surface protective barrier allows the consolidation of the lesion and the inhibition of further progression of demineralization. The homogeneous mineral sediment in fTCP is likely to play an important role as a leak-proof seal against acid dissolution compared with the defective, porous surface precipitation in CPP-ACPF.
Although saliva was reported to induce a certain amount of remineralization (Jayarajan et al., 2011), it cannot by itself increase the levels of calcium and phosphate release. For mineral deposition to occur within the body of the lesion, calcium and phosphate ions must first penetrate the surface layer of enamel. Mineral deposition must occur in a controlled and directed fashion to be efficacious, either targeting the lesion directly or elevating calcium concentrations in the oral reservoirs (Lynch and Smith, 2012). This can be attributed to the fact that the control, which was untreated and stored in artificial saliva for 10 days, showed no decrease in the lesion depth and further progression after the acid attack in the current study.
Remineralization in vitro may be quite different from a dynamic complex biological system which usually occurs in oral cavity. The limitation of this study relate to the formation of enamel lesions, the duration of remineralization, and the method used in further acid attack. The outermost demineralized layer was removed and cavity was created after the lesion formation in the CLSM images. It seems that the surface of the lesion became fragile and was susceptible to damage during the topical application of remineralizing agents since the artificially demineralized enamels were vulnerable to the mechanical stress. The observation time was limited to 10 d. Because remineralization is a long-term process, this short-term in vitro study is unable to demonstrate the long-term capabilities of the remineralizing agents. Furthermore, the dimineralizing solution was used for the further acid challenge in place of the pH cycling protocol, which is usually used in the formation of artificial caries lesions. This might exaggerate the dissolution of the surface layer and lesion propagation in CPP-ACPF. With the use of low fluoride concentration as is present in CPP-ACPF (0.2% or 900 ppm of NaF), there was a complex localization of free calcium phosphate and fluoride ion activities, which helped in maintaining a state of supersaturation by suppressing demineralization (Holler et al., 2002). Hence, caution must be used when generalizing the results directly to the clinical situations and future studies should be conducted on the pH cycling model with non-cavitated enamel lesions to clarify the long-term effects of remineralizing agents.
Conclusions
Within the limitation of this in vitro study, it can be concluded that topical application of the remineralizing agents on artificial enamel lesions can inhibit further demineralization of the lesions and fTCP seems to be more advantageous than CPP-ACPf for this purpose. The lesion should be remineralized homogeneously not only the surface but also the subsurface lesion body. Calcium, phosphate, and fluoride ions must first penetrate the surface layer of enamel and mineral gain within the body of the lesion should occur through the diffusion of the ions.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012-0009327)
References
- P Barkvoll, G Embery, G Rolla, Studies on the interaction between sodium lauryl sulfate and hydroxyapatite using Fourier transformed infrared spectroscopy, J Biol Buccale, (1988a), 16, p75-79.
-
P Barkvoll, G Rolla, F Lagerlof, Effect of sodium lauryl sulfate on the deposition of alkali-soluble fluoride on enamel in vitro, Caries Res, (1988b), 22, p139-144.
[https://doi.org/10.1159/000261094]

-
SM Behnan, AO Arruda, C Gonzalez-Cabezas, W Sohn, MC Peters, In-vitro evaluation of various treatments to prevent demineralization next to orthodontic brackets, Am J Orthod Dentofacial Orthop, (2010), 138(712), pe1-7, discussion 712-713.
[https://doi.org/10.1016/j.ajodo.2010.05.014]

-
F Cai, P Shen, MV Morgan, EC Reynolds, Remineralization of enamel subsurface lesions in situ by sugar-free lozenges containing casein phosphopeptide- amorphous calcium phosphate, Aust Dent J, (2003), 48, p240-243.
[https://doi.org/10.1111/j.1834-7819.2003.tb00037.x]

-
NJ Cochrane, F Cai, NL Huq, MF Burrow, EC Reynolds, New approaches to enhanced remineralization of tooth enamel, J Dent Res, (2010), 89, p1187-1197.
[https://doi.org/10.1177/0022034510376046]

-
AM Geiger, L Gorelick, AJ Gwinnett, BJ Benson, Reducing white spot lesions in orthodontic populations with fluoride rinsing, Am J Orthod Dentofacial Orthop, (1992), 101, p403-407.
[https://doi.org/10.1016/0889-5406(92)70112-N]

- C Gonzalez-Cabezas, M Fontana, AJ Dunipace, Y Li, GM Fischer, HM Proskin, GK Stookey, Measurement of enamel remineralization using microradiography and confocal microscopy, A correlational study. Caries Res, (1988), 32, p385-392.
- BE Holler, KH Friedl, H Jung, KA Hiller, G Schmalz, Fluoride uptake and distribution in enamel and dentin after application of different fluoride solutions, Clin Oral Investig, (2002), 6, p137-144.
-
J Jayarajan, P Janardhanam, P Jayakumar, Efficacy of CPP-ACP and CPP-ACPF on enamel remineralization - an in vitro study using scanning electron microscope and DIAGNOdent, Indian J Dent Res, (2011), 22, p77-82.
[https://doi.org/10.4103/0970-9290.80001]

-
RL Karlinsey, AC Mackey, Solid-state preparation and dental application of an organically modified calcium phosphate, J Mater Sci, (2009), 44, p346-349.
[https://doi.org/10.1007/s10853-008-3068-1]

-
RL Karlinsey, AC Karlinsey, ER Walker, KE Frederick, Surfactant-modified beta-TCP: structure, properties, and in vitro remineralization of subsurface enamel lesions, J Mater Sci Mater Med, (2010), 21, p2009-2020.
[https://doi.org/10.1007/s10856-010-4064-y]

-
A Kawska, J Brickmann, R Kniep, O Hochrein, D Zahn, An atomistic simulation scheme for modeling crystal formation from solution, J Chem Phys, (2006), 124, p024513.
[https://doi.org/10.1063/1.2145677]

-
S Lata, NO Varghese, JM Varughese, Remineralization potential of fluoride and amorphous calcium phosphate-casein phospho peptide on enamel lesions: An in vitro comparative evaluation, J Conserv Dent, (2010), 13, p42-46.
[https://doi.org/10.4103/0972-0707.62634]

-
JK Liu, TM Lee, IH Liu, Effect of loading force on the dissolution behavior and surface properties of nickel-titanium orthodontic archwires in artificial saliva, Am J Orthod Dentofacial Orthop, (2011), 140, p166-176.
[https://doi.org/10.1016/j.ajodo.2010.03.031]

-
RJ Lynch, SR Smith, Remineralization agents - new and effective or just marketing hype?, Adv Dent Res, (2012), 24, p636-7.
[https://doi.org/10.1177/0022034512454295]

-
MS Mathews, BT Amaechi, K Ramalingam, RA Ccahuana-Vasquez, IP Chedjieu, AC Mackey, RL Karlinsey, In situ remineralisation of eroded enamel lesions by NaF rinses, Arch Oral Biol, (2012), 57, p525-530.
[https://doi.org/10.1016/j.archoralbio.2011.10.010]

-
PK Mensinkai, RA Ccahuana-Vasquez, I Chedjieu, BT Amaechi, AC Mackey, TJ Walker, DD Blanken, RL Karlinsey, In situ remineralization of white-spot enamel lesions by 500 and 1,100 ppm F dentifrices, Clin Oral Investig, (2012), 16, p1007-1014.
[https://doi.org/10.1007/s00784-011-0591-2]

-
S Paris, H Meyer-Lueckel, J Mueller, M Hummel, AM Kielbassa, Progression of sealed initial bovine enamel lesions under demineralizing conditions in vitro, Caries Res, (2006), 40, p124-129.
[https://doi.org/10.1159/000091058]

-
EI Pearce, DG Nelson, Microstructural features of carious human enamel imaged with back-scattered electrons, J Dent Res, (1989), 68, p113-118.
[https://doi.org/10.1177/00220345890680020301]

-
AM Pfarrer, RL Karlinsey, Challenges of implementing new remineralization technologies, Adv Dent Res, (2009), 21, p79-82.
[https://doi.org/10.1177/0895937409335643]

-
EC Reynolds, CJ Cain, FL Webber, CL Black, RF Riley, IH Johnson, JW Perich, Anticariogenicity of calcium phosphate complexes of tryptic casein phosphopeptides in the rat, J Dent Res, (1995), 74, p1272-1279.
[https://doi.org/10.1177/00220345950740060601]

-
EC Reynolds, Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions, J Dent Res, (1997), 76, p1587-1595.
[https://doi.org/10.1177/00220345970760091101]

-
EC Reynolds, F Cai, P Shen, GD Walker, Retention in plaque and remineralization of enamel lesions by various forms of calcium in a mouthrinse or sugar-free chewing gum, J Dent Res, (2003), 82, p206-211.
[https://doi.org/10.1177/154405910308200311]

-
EC Reynolds, F Cai, NJ Cochrane, P Shen, GD Walker, MV Morgan, C Reynolds, Fluoride and casein phosphopeptide-amorphous calcium phosphate, J Dent Res, (2008), 87, p344-348.
[https://doi.org/10.1177/154405910808700420]

- N Rodriguez-Hornedo, D Murphy, Surfactantfacilitated crystallization of dihydrate carbamazepine during dissolution of anhydrous polymorph, J Pharm Sci, (2004), 93, p449-460.
-
RK Rose, Effects of an anticariogenic casein phosphopeptide on calcium diffusion in streptococcal model dental plaques, Arch Oral Biol, (2000), 45, p569-575.
[https://doi.org/10.1016/S0003-9969(00)00017-0]

- RJ Sullivan, J Masters, R Cantore, A Roberson, I Petrou, M Stranick, H Goldman, B Guggenheim, A Gaffar, Development of an enhanced anticaries efficacy dual component dentifrice containing sodium fluoride and dicalcium phosphate dihydrate, Am J DentSpec No:3A-11A, (2001).
- JM ten Cate, J Arends, Remineralization of artificial enamel lesions in vitro: III, A study of the deposition mechanism. Caries Res, (1980), 14, p351-358.
- JM ten Cate, In vitro studies on the effects of fluoride on de- and remineralization, J Dent Res 69 Spec No:614-619; discussion 634-636, (1990).
-
C Trairatvorakul, S Kladkaew, S Songsiripradabboon, Active management of incipient caries and choice of materials, J Dent Res, (2008), 87, p228-232.
[https://doi.org/10.1177/154405910808700301]

- Z Amiad, Calcium phosphates in biological and industrial systems, Norwell: Springer, (1998), p1-20.
- DJ White, The application of in vitro models to research on demineralization and remineralization of the teeth, Adv Dent Res 9:175-193; discussion 194-197, (1995).
-
DR Willmot, White lesions after orthodontic treatment: does low fluoride make a difference?, J Orthod 31:235-242; discussion 202, (2004).
[https://doi.org/10.1179/146531204225022443]