Effects of surface treatments and 2% chlorhexidine application on shear bond strength of resin-modified glass ionomer to dentin
본 연구는 두 가지 표면처리 방법과 2% chlorhexidine의 적용이 레진 강화형 글라스 아이오노머의 상아질 결합력에 미치는 영향을 평가하였다. 표면처리 방법의 종류, 2% chlorhexidine의 처리 유무에 따라 총 4개의 군으로 나누었다. 1군: 10% polyacrylic acid로 상아질 표면을 처리, 2군: G-aenial bond (한단계 자가부식 접착제)로 표면처리, 3군: 10% polyacrylic acid로 표면처리 후 2% chlorhexidine 적용, 4군: 2% chlorhexidine 적용 후 G-aenial bond를 처리. 처리된 상아질 표면에 레진 강화형 글라스 아이오노머(Fuji Ⅱ LC)를 충전하고 광중합한 후 전단결합강도를 측정하고 일원분산분석과 Scheffé 검사를 이용하여 통계 분석하였다. 상아질의 표면처리를 10% polyacrylic acid로 시행한 1, 3군의 전단결합강도 값이 one step self-etching adhesive로 표면처리를 한 2, 4군에 비해 유의하게 낮게 나타났다 (p<0.05). 2% chlorhexidine의 적용은 레진 강화형 글라스 아이오노머의 상아질에 대한 전단결합강도에 유의한 영향을 주지 않았다 (p>0.05).
Keywords:
Chlorhexidine, Resin-modified glass-ionomer, Shear bond strength, Surface treatmentINTRODUCTION
Glass ionomers were developed in the 1970s by Wilson and Kent (Wilson and Kent, 1972). The advantages of glass ionomers are potential anticariogenic effect, favorable coefficient of thermal expansion, and releasing fluoride into the surrounding tooth structure (Swift, 1989; Mount, 1991). However, resistance to wear and strength of glass ionomers are low compared with those of composite resin or amalgam. In an attempt to improve the physical properties and esthetic qualities while maintaining the positive characteristics of conventional glass ionomers, resin-modified glass ionomer (RMGI) materials have been introduced.
RMGI restorative techniques generally use conditioner as a part of their bonding procedure (Eileen et al., 2002). During this procedure the role of the conditioner involves effective removal of the smear layer, providing for good wetting of the surface required condition for good bonding and finally improves chemical bond between RMGI materials and tooth structure (Akinmade and Nicholson, 1993). However, Eileen et al.(2002) suggested that the possibility of enhanced bonding by etching enamel due to mono mers containing RMGI materials. They contended that micromechanical bonding may play a role in bonding of RMGI to enamel. In addition, a number of studies proposed that bond strength of RMGI to dentin could be improved by surface treatment with a self-etching bonding system (Pereira et al., 1998; Besnault et al., 2004; Coutinho et al., 2006; El-Askary and Nassif, 2010; Elisabeth and Jean-Pierre, 2011).
On the other hands, in restorative dentistry, one of the problems is remained bacteria in prepared cavity and it may cause recurrent caries and the toxins produced by such bacteria penetrate into the pulp chamber and result in pulpal inflammation (Say et al., 2004). An effective alternative method to this problem is the irrigation of the prepared cavity with cavity disinfectant agents such as chlorhexidine digluconate (CHX).
However, a potential problem in the application of CHX on dentin is the possibility of an adverse effect on the bond strength of the following restorative materials. Despite the concern, several researches have commended the use of CHX as the cavity disinfectant because it did not weaken bond strength (Loguercio et al., 2009; Chang and Shin, 2010; Jang et al., 2010). In a study about the effect of 2% CHX application on microtensile bond strength of resin composite to dentin using one step self etching adhesive, CHX did not influence on the microtensile bond strength (Jang et al., 2010). In addition, a research on the effect of CHX treatment on microtensile bond strength to dentin after thermocycling identified beneficial effects of CHX in the preservation of dentin bond strength (Chang and Shin, 2010). Loguercio et al. (2009) suggested that CHX application may preserve resin-dentin bond from degradation. They proposed that CHX inhibit the releasing and activating of matrix metalloproteinases (MMPs) which play a role in degradation of collagen fibers in the hybrid layer (Loguercio et al., 2009; Chang and Shin, 2010). According to the study of Rose Wadenya et al. (2009), application of CHX increased the shear bond strength of glass ionomer to dentin, but was not statistically significant. To date, only a few studies have investigated the effect of CHX application on shear bond strength (SBS) of RMGI to dentin.
Thus, the purpose of this in vitro study is to evaluate the effects of two different surface treatments (conditioning with 10% polyacrylic acid, self-etching adhesive) and the influence of CHX application on shear bond strength (SBS) of RMGI to dentin.
MATERIALS AND METHODS
1. Tooth preparation
Forty caries-free human third molars were used in this study. The teeth were cleansed of soft tissue debris and stored in distilled water before use. The teeth were wet ground on a laboratory trimmer to expose approximately 10 mm of flat dentin surface. The sections of the teeth including the roots were embedded in autopolymerizing acrylic resin (Tokuso curefast; Tokuyama, Tokyo, Japan) to form cylinders 20 mm in diameter and 25 mm high. To expose flat dentin surface, a 600 grit silicon carbide paper was used under running water.
2. Specimen Preparation and Restorative Procedures
The teeth (n=40) were randomly divided into four groups (n=10), according to the type of surface treatment and the application of CHX. For group 1, the dentin surfaces were conditioned with 10% polyacrylic acid (Dentin conditioner; GC Corporation, Tokyo, Japan) for 20 seconds, followed by thorough washing and air-drying. For group 2, G-aenial bond (GC Corporation, Tokyo, Japan) was applied using microbrush for 10 seconds, dried for 5 seconds and light-cured for 10 seconds with a light-emitting diode (LED) visible lightpolymerizing unit (Elipar S10; 3M ESPE, Seefeld, Germany). For group 3, 2% CHX solution applied for 60 seconds and dried for 10 seconds after 10% polyacrylic acid conditioning as for group 1 to prevent removal of CHX during washing and air-drying procedure. For group 4, G-aenial bond was applied after using CHX as previously described. Table 1 is a summary of four groups used in this study.
Following surface treatment, two semicylindric Teflon molds were placed on the dentin in order to build RMGI cylinders with a diameter of 3 mm and a height of 2 mm. The RMGI (GC Fuji Ⅱ LC Capsule A2; GC Corporation, Tokyo, Japan) was inserted into the mold in bulk, light-cured with Elipar S10 LED curing light for 20 seconds. The materials used in current study are given in Table 2.
3. Shear bond strength testing
All specimens were stored in distilled water at room temperature for 24 hours. Then, the specimens were placed on a universal testing machine (Instron 3345; Instron Corporation, Canton, MA, USA) at a crosshead speed of 1 mm/min for measurement of shear bond strength. The shear bond strength of RMGI to dentin was recorded in Newtons (N) and calculated in MPa taking into account the cross-sectional area of the RMGI build-up.
4. Statistical analysis
Statistical analysis of the shear bond strength between groups was performed with one-way analysis of variance (ANOVA) and Scheffé test was used for post hoc multiple comparison. Statistical analysis was carried out with the SPSS 15.0 software (SPSS, Chicago, IL, USA) and statistical significance was set at p < 0.05 in this study.
RESULTS
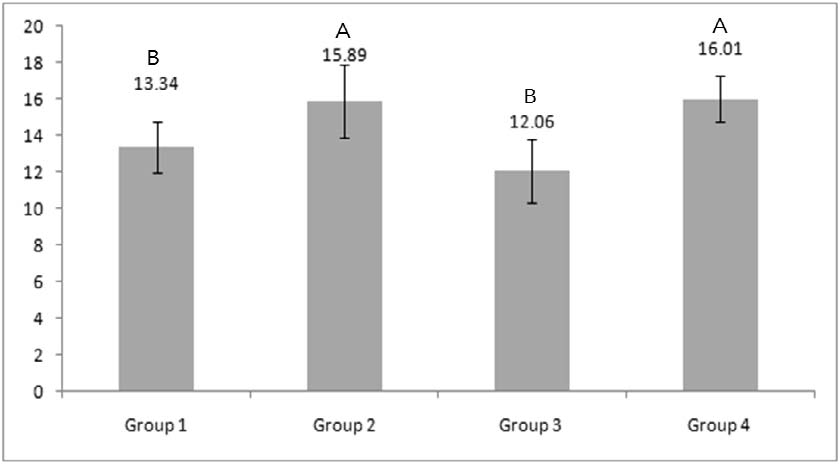
The means and standard deviations (SD) of the shear bond strength for all groups are shown in Figure 1.
The bond strengths of groups 2 and 4 were significantly higher than groups 1 and 3 (p<0.05), there being no differences between the former two, and between the latter two (p>0.05).
DISCUSSION
In the present study, the application of one step self-etching adhesive, G-aenial bond significantly improved shear bond strength of RMGI to dentin than conditioning with 10% polyacrylic acid.
It is well known that glass ionomers particularly conventional glass ionomer, bond to tooth structure by an ionic interaction with the mineral phase. However, Vougiouklakis G et al. (1982) proposed that the bond mechanism was, at least in part, due to penetration of glass ionomer into the demineralized dentin and tubules, forming micromechanical bonds. Also, Eileen et al. (2002) suggested that microporosity produced by acidic materials in the tooth surface could contribute to either increased surface area for chemical bonding or micromechanical bonding through polymer penetration and the relatively high bond strengths obtained with the RMGI groups were due to micromechanical bonding.
Furthermore, many studies have shown that adherence values of RMGI to dentin can be improved by surface treatment with a self-etching bonding system (Pereira et al., 1998; Besnault et al., 2004; Coutinho et al., 2006). Besnault et al. (2004) found that the combination of self-etching adhesive with RMGI (Fuji Ⅱ LC) has resulted in a significant increase (from 50% to 130%) of the shear bond strength compared to the procedure using the manufacture-supplied cavity conditioner. Also, Elisabeth et al. (2011) confirmed that selfetching adhesive in association with the Fuji Ⅱ LC increased the shear bond strength very significantly, even in the case of water or saliva contamination.
G-aenial bond is used as a self-etching adhesive in our study. According to the manufacturer's guidelines, it has strong adhesive strength overcoming the limitations of one step bonding materials by optimization combination of phosphate ester monomer and 4-methacryloxyethyl trimellitic acid (4-MET).
The results of this study coincide with the outcomes of the previous study in which the use of self-etching adhesive improves the RMGI-dentin bond strength. For achieving a better bond strength, application of selfetching adhesive on dentin prior to build-up of RMGI is recommended by findings of the current study.
CHX is widely used as an antimicrobial agent that possesses a broad spectrum of activity against oral bacteria and has been shown to be effective in reducing cariogenic bacteria (Attin et al., 2003).
In the present study, treatment of CHX did not affect the bond strength of RMGI to dentin. Rose et al, (2011) found that cleaning of dentin surface with CHX resulted in increased bond strength of high-density glass ionomer cements to dentin, but the differences were not statistically significant. They anticipated that CHX improves the bond strength of glass ionomer cement to dentin because of its strong, positive ionic charge, which makes it bind to phosphate groups, hydroxyapatite and amino acids. However, in their study, CHX exerted no significant influence on the bond strength between dentin and glass ionomer cements as this study.
In the bond of resin-dentin interface, another important aspect related to the use of CHX is the influence of matrix metalloproteinases (MMPs). Failure of the bond is predominantly caused by degradation of the hybrid layer at dentin-resin interface and also the degradation of dentin collagen fibers (Takahashi et al., 2002; Hashimoto et al., 2003). It seems the release and activation of MMPs during dentin bonding procedures is responsible for the degradation of collagen fibers which have not been completely covered in the hybrid layer (Hashimoto et al., 2002; Nishitani et al., 2006). CHX has effectively inhibited MMP-2, -8, and -9 activities directly at low concentrations (Gedron et al., 1999). Many of studies showed that the application of CHX preserved dentin-resin interfaces over a long period of time (Loguercio et al., 2009; Mobarak, 2011; Kang et al., 2012). Therefore, further studies are needed to evaluate whether the CHX will have any effect on bonding durability of RMGI to dentin for the long term.
Within the limitations of this study, it may conclude that the treatment with one step self-etching adhesive improves the bond strength of RMGI to dentin than conditioning with 10% phosphoric acid. Furthermore, the application of CHX is recommended because CHX has antimicrobial characteristic and does not adversely affect on the bond strength of RMGI to dentin. Further studies are needed to investigate the long term effects of CHX on bonding durability of RMGI to dentin.
CONCLUSION
Based on the results of this in vitro study, following conclusions were drawn. The use of self-etching adhesive improved the RMGI-dentin bond strength than treatment with 10% polyacrylic acid and the application of 2% CHX had exerted no influence on the shear bond strength.
Acknowledgments
This study was supported by Clinical Research Grant, Pusan National University Dental Hospital.
References
- AO Akinmade, JW Nicholson, Review: Glassionomer cements as adhesives. Part I Fundamental aspects and their clinical relevance, J Mater Sci Mater Med, (1993), 4, p95-101.
-
R Attin, A Tuna, T Attin, E Brunner, MJ Noack, Efficacy of differently concentrated chlorhexidine varnishes in decreasing mutans streptococci and lactobacilli counts, Arch Oral Biol, (2003), 48, p503-509.
[https://doi.org/10.1016/S0003-9969(03)00093-1]

- C Besnault, JP Attal, D Ruse, M Ruse, Selfetching adhesives improve the shear bond strength of a resin-modified glass-ionomer cement to dentin, J Adhes Dent, (2004), 6, p55-59.
-
YE Chang, DH Shin, Effect of chlorhexidine application methods on microtensile bond strength to dentin in Class I cavities, Oper Dent, (2010), 35(6), p618-623.
[https://doi.org/10.2341/09-360-L]

-
E Coutinho, K Van Landuyt, J De Munck, A Poitevin, Y Yoshida, S Inoue, M Peumans, K Suzuki, P Lambrechts, B Van Meerbeek, Development of a self-etch adhesive for resin-modified glass ionomers, J Dent Res, (2006), 85, p349-353.
[https://doi.org/10.1177/154405910608500413]

- Glasspoole Eileen A, Glasspoole, Erickson Robert L, Davidson Carel L, Effect of surface treatments on the bond strength of glass ionomers to enamel, Dent Mater, (2002), 18, p454-462.
- F El-Askary, M Nassif, Bonding Nano-filled resinmodified glass ionomer to dentin using different self-etch adhesive, Oper Dent, (2010), 36(4), p413-421.
- Dursun Elisabeth, Attal Jean-Pierre, Combination of a self-etching adhesive and a resin-modified glass ionomer: Effect of water and saliva contamination on bond strength to dentin, J Adhes Dent, (2011), 13, p439-443.
- R Gedron, D Grenier, T Sorsa, D Mayrand, Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine, Clin Diagn Lab Immunol, (1999), 6(3), p437-439.
-
M Hashimoto, H Ohno, H Sano, FR Tay, M Kaga, Y Kudou, H Oguchi, Y Araki, M Kubota, Micromorphological changes in resin-dentin bonds after 1 year of water storage, J Biomed Mater Res, (2002), 63, p306-311.
[https://doi.org/10.1002/jbm.10208]

-
M Hashimoto, FR Tay, H Ohno, H Sano, M Kaga, C Yin, H Kumagai, Y Kudou, M Kubota, H Oguchi, SEM and TEM analysis of water degradation of human dentinal collagen, J Biomed Mater Res B Appl Biomater, (2003), 66, p287-298.
[https://doi.org/10.1002/jbm.b.10560]

-
SH Jang, B Hur, HC Kim, YH Kwon, JK Park, Effect of 2% chlorhexidine application on microtensile bond strength of resin composite to dentin using one-step self-etch adhesives, J Korean Acad Conserv Dent, (2010), 35(6), p486-491.
[https://doi.org/10.5395/JKACD.2010.35.6.486]

-
HJ Kang, HJ Moon, DH Shin, Effect of different chlorhexidine application times on microtensile bond strength to dentin in Class Ⅰ cavities, Restor Dent Endod, (2012), 37(1), p9-15.
[https://doi.org/10.5395/rde.2012.37.1.9]

-
AD Loguercio, R Stanislawczuk, LG Polli, JA Costa, MD Michel, A Reis, Influence of chlorhexidine digluconate concentration and application time on resin–dentin bond strength durability, Eur J Oral Sci, (2009), 117, p587-596.
[https://doi.org/10.1111/j.1600-0722.2009.00663.x]

-
EH Mobarak, Effect of chlorhexidine pretreatment on bond strength durability of caries-affected dentin over 2-year aging in artificial saliva and under simulated intrapulpal pressure, Oper Dent, (2011), 36(6), p649-660.
[https://doi.org/10.2341/11-018-L]

- GJ Mount, Adhesion of glass-ionomer cement in the clinical environment, Oper Dent, (1991), 16, p141-148.
-
Y Nishitani, M Yoshiyama, B Wadgaonkar, L Breschi, F Mannello, A Mazzoni, RM Carvalho, L Tjäderhane, FR Tay, DH Pashley, Activation of gelatinolytic/ collagenolytic activity in dentin by selfetching adhesives, Eur J Oral Sci, (2006), 114, p160-166.
[https://doi.org/10.1111/j.1600-0722.2006.00342.x]

-
PN Pereira, T Yamada, S Inokoshi, MF Burrow, H Sano, J Tagami, Adhesion of resin-modified glass ionomer cements using resin bonding systems, J Dent, (1988), 26, p479-485.
[https://doi.org/10.1016/S0300-5712(97)00059-6]

- Wadenya Rose, Menon Sandhya, Mante Francis, Effect of chlorhexidine disinfectant on bond strength of glass ionomer cement to dentin using Atraumatic restorative treatment, NY State Dental J, (2011), 77(1), p23-26.
- EC Say, F Koray, B Tarim, M Soyman, T Gülmez, In vitro effect of cavity disinfectants on the bond strength of dentin bonding systems, Quintessence Int, (2004), 35(1), p56-60.
- Jr Swift EJ, Effect of glass ionomers on recurrent caries, Oper Dent, (1989), 14, p40-43.
- A Takahashi, S Inoue, C Kawamoto, R Ominato, T Tanaka, Y Sato, In vivo long-term durability of the bond to dentin using two adhesive systems, J Adhes Dent, (2002), 4, p151-159.
-
G Vougiouklakis, DC Smith, S Lipton, Evaluation of the bonding of cervical restorative materials, J Oral Rehab, (1982), 9, p231-51.
[https://doi.org/10.1111/j.1365-2842.1982.tb01014.x]

-
AD Wilson, BE Kent, A new translucent cement for dentistry. The glass ionomer cement, Br Dent J, (1972), 132, p133-135.
[https://doi.org/10.1038/sj.bdj.4802810]