Effects of Bioactive Glass on Microhardness of Bleached Enamel Surface

Abstract
The purpose of this study was to examine effects of bioactive glass on Vickers hardness of bleached enamel surface. Enamel specimens were bleached with 15% carbamide peroxide (CP) for 14days. After bleaching, Artificial saliva (AS), 45S5 bioactive glass (BAG) and fluoride varnish (FV) are applied each specimens (n=10). The Vickers hardness of the specimens was measured before and after the bleaching, after the remineralized treatment at 1 day and after 7 and 14 days. The Vickers hardness on enamel surface after bleaching decreased statistically (p<0.05) compared to before bleached enamel surface. The Vickers hardness increased significantly in the BAG and FV groups compared to the AS group values at 1 day and 7 days after remineralization (P<0.05). 45S5 bioactive glass rapidly increase Vickers hardness of the bleached enamel surface.
초록
본 연구는 미백제를 적용한 법랑질 표면에 Bioactive glass 45S5를 적용 시 법랑질 표면의 비커스 경도에 미치는 영향을 파악하고자 하였다. 건전한 인간 소구치를 이용하여 법랑질 표면을 포함한 시편 (3×3×2 mm3)을 제작하였다. 이후, 15% Carbamide Peroxide를 사용하여 14일 동안 하루에 8시간 미백하고 이후 시간은 인공타액에 보관하였다. 시편을 세 군으로 나누어(n=10), 각각 재광화 처치를 하지 않고 인공 타액에 저장한 군(AS군), Bioactive glass 45S5로 4시간 재광화 처리한 군(BAG군), 불소 제품인 Clinpro™ white varnish로 4시간 처리한 군(FV군)으로 처치하였다. 미백 전후, 재광화 처치 후 1일, 7일, 14일 때 법랑질 표면의 비커스 경도를 각각 측정하였다.
비커스 경도는 미백 전에 비하여 미백 후 유의하게 감소하였다(p<0.05). 재광화 처치 1일 때, BAG와 FV군은 AS군에 비하여 비커스 경도가 유의하게 증가하였다(p<0.05). 재광화 처치 1일과 7일 BAG군은 AS군에 비해 더 높은 비커스 경도를 보였으나, 14일에는 통계학적으로 유의한 차이를 나타내지 않았다(p>0.05). 미백제에 의해 감소된 법랑질 표면의 비커스 경도는 Bioactive glass 처리시 빠르게 증가한다.
Keywords:
Bleaching, 45S5 bioacitive glass, Remineralization키워드:
미백, Bioactive glass 45S5, 재광화INTRODUCTION
Teeth whitening is the most popular method of brightening teeth and home vital bleaching is one method of whitening. This method uses individual whitening trays to put carbamide peroxide (CP) or hydrogen peroxide (HP) on a tray that the patient wears during normal sleeping so that the teeth can be whitened (Haywood VB, 1991). Teeth are bleached by the oxidation and reduction of free radicals, such as oxygen and perhydroxyl in hydrogen peroxide. Because there are no electrons in the outermost atomic layer, oxygen (O•) and perhydroxyl (HO2•) free radicals are highly reactive and electrophilic. They can spread throughout the enamel and dentin matrix and attack stable endogenous pigment molecules. After this interaction, the endogenous pigment is composed of an unsaturated organic polymer that becomes a less complex molecular structure that is then converted to a smaller, lighter molecule than the original molecule (Rodrigues J.A et al, 2007). The bleaching of teeth occurs as a result.
Despite the widespread success of tooth bleaching products in whitening teeth, there is no general consensus regarding the possible negative effects on the enamel (Joiner A, 2006). On the other hand, in many articles, tooth whitening agents have been reported to affect the fracture toughness, chemical composition, and surface morphology of the tooth surfaces (Sa Y et al, 2013; Basting RT et al, 2003). Some studies have observed surface changes by scanning electron microscopy as well as reduced surface hardness. In a SEM study, Ernst CP et al found significant surface alterations in enamel topography following enamel bleaching (Ernst CP et al, 1996; Josey AL et al, 1996). An in vitro study by Flaitz and Hicks showed that various concentrations of carbamide peroxide remove mineral structures from enamel, causing morphological changes in various morphologies and strengths and reaching the underground surface (Flaitz CM et al, 1996). Therefore, damage to the enamel surface by tooth whitening agents should be minimized. In many studies, this damage can be reduced by applying a remineralizing agent to the affected tooth surface after bleaching. Among the remineralizing agents available, many advantages in the use of fluorine during or after whitening have been reported (Lewinstein I et al, 2004; Bizhang M et al, 2006; Magalhães A et al, 2011).
Recently, bioactive glass has been introduced as one of the other remineralizing agents. This material has different proportions of oxides of calcium, sodium, phosphorus, and silica depending on the bioactivity. In vivo, bioactive glass has been reported to form hydroxyapatite layers. 45S5 bioactive glass, which was developed by Hench et al., consists of 45% SiO2, 24.5 Na2O, 24.5% CaO and 6% P2O5 by weight (Hench LL, 1971). Several studies have suggested that bioactive glass blocks the dentinal tubules through the apatite layer, inhibits demineralization, and promotes remineralization (Curtis AR et al, 2010; Vollenweider M et al, 2007). These layers were reported to adhere tightly to the dentine tubules and are resistant to acid and brushing (Andersson O¨H & Kangasniemi I,1991). It has been introduced a commercial bioactive glass such as NovaMin, used to promote enamel remineralization and treat tooth sensitivity (Burwell A et al, 2010). However, several bioactive glass containing toothpastes were examined for evaluating their overall ability to repair the enamel surface following bleaching (Gjorgievska E & Nicholson JW, 2010). Also, because the marketed dentifrice ingredient contains a small amount of the bioactive glass, few studies have evaluated the effects of the bioactive glass itself. Therefore, this study evaluated that the changes to the enamel microhardness after bleaching and the effects of subsequent applications of bioactive glass on bleached enamel. The null hypotheses ested in the study were that 1) bleaching treatment using 15% carbamide peroxide on enamel surface would not change hardness of enamel and 2) remineralization treatment using bioactive glass would not affect bleached enamel surface.
MATERIALS AND METHODS
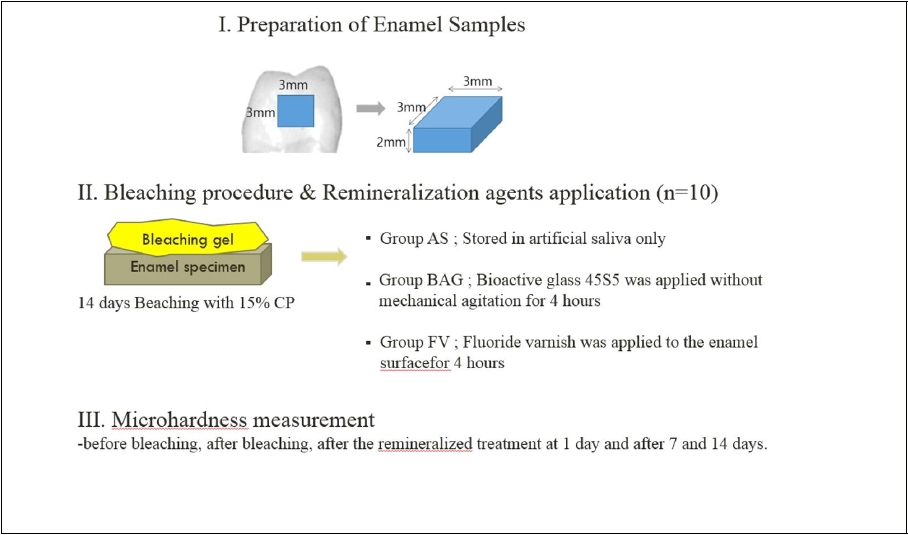
1. Preparation of Enamel Specimens
Ten freshly extracted noncarious human premolars were selected. The ethics committee of the institution where the experiment was performed approved the protocol (PNUDH-2016-026). The teeth were cleaned thoroughly to remove any deposits with a scaler. The roots of the teeth were removed at the cementoenamel junction with a low speed diamond saw (Isomet 1000; Buehler, Lake Bluff, IL, USA). The crown part was sectioned mesiodistally and buccolingually into four parts. Each quarter of the crown was ground on the dentin side, leaving a 2-mm-thick dentin layer. Forty specimens were obtained and finally 30 specimens were selected after surface confirmation using a microscope. The sections were embedded in acrylic resin with a 3×3 mm window on the exposed enamel surface and then ground and polished with 600-grit carborundum paper discs (Buehler Met II grinding paper; Buehler) in a polishing machine (Ecomet 3; Variable speed grinding polisher; Buehler). Subsequently the specimens were ultrasonically cleaned for 5 min in distilled water bath, and inspected under a stereomicroscope (Leica, Wetzlar, Germany) to exclude those with cracks or defects. Finally, the specimens were stored at Hank’s balanced salts solution until use.
2. Bleaching Procedures
The specimens were assigned to a bleaching agent containing 15% CP (Opalescence 15% CP Ultradent Products; South Jordan, UT, USA). Prior to bleaching, the enamel surfaces were dried with cotton pellets. The enamel surfaces of the specimens were then covered with a 1 mm layer of the bleaching gel. The specimens were bleached for eight hours and kept in a humid atmosphere at 37℃. After each bleaching procedure, the gel was removed with a cotton pellet and the specimens were washed and dried with blowing air for five seconds. The specimens were then stored in artificial saliva at 37℃. The specimens were bleached for 14 consecutive days. Immediately after the 14 days of treatment, the specimens were assigned into three groups containing 10 specimens according to the remineralizing agent. Compositions of materi are described in Table 1.
- 1. Group AS: Stored in artificial saliva only(Negative control – Xerova solution; Kolmar Korea Co., Ltd., Sejong, Korea).
- 2. Group BAG: Bioactive glass 45S5(Aladdin Industrial Corporation, Shanghai, China) was prepared as a slurry (BAG : distill water ratio = 1:1), and applied without mechanical agitation for 4 hours
- 3. Group FV: Fluoride varnish (Clinpro™; 3M corp., St. Paul, MN, USA) was applied to the enamel surface and left undisturbed for 4 hours, as recommended in the manufacturer’s guidelines(Positive control).
After this period, fluoride varnish was carefully removed from the surface using acetone and a scalpel blade, taking care to avoid touching the enamel surface. Bioactive glass was removed with a cotton pellet and the specimens were washed and dried with blowing air for five seconds. Complete removal of the layer was confirmed microscopically (×40).
3. Microhardness test
The surface microhardness (measured as the Vickers hardness number [VHN]) was measured at the baseline, i.e., before the bleaching treatments, directly after bleaching treatment at 14 days, after the remineralized treatment at 1 day and after 7 and 14 days. The surface micorhardness of the enamel specimens was measured using a microhardness tester (MVK-H1; Akashi Co., Tokyo, Japan) loaded to 300g with an indentation time of 5 seconds (Chuenarrom C et al, 2009; Alankrita C et al, 2013). Three indentations were performed on the center of each specimen with a distance of 100μm between them and the results were averaged.
4. Statistical analysis
Statistical analysis was performed using statistical software (SPSS 18.0; SPSS Inc., Chicago, IL, USA). The microhardness values were compared according to the bleaching agents, the subsequent application of remineralization treatments, and the different time intervals using a two-way ANOVA/ Duncan test (p<0.05). The microhardness values of each group were analyzed using a one-way ANOVA/ Duncan test (α<0.05) for the bleaching time factor, within the group. Statistical significance was set to p<0.05 for all tests.
RESULTS
Table 2 lists the microhardness values before and after the bleaching procedures in each group. A significant difference in the microhardness values was observed according to the day (P<0.05) and among the groups (P<0.05). A significant difference in the interaction effect was observed between the day and remineralization (P<0.05). In all three groups, the microhardness values decreased after bleaching. The micorhardness values increased significantly in the BAG and FV groups compared to the AS group values at 1 day and 7 days after remineralization (P<0.05). At 1 day and 7 days after remineralization, the BAG group exhibited higher microhardness than the AS group, but at 14 days after remineralization, the differences were not significant (P=0.069).
DISCUSSION
The microhardness is a relatively simple and reliable method compared to other measurements in relation to the changes in the mineral content of the hard tissue (Rodrigues JA et al, 2005; Basting RT et al, 2003). Mineral change on the enamel surface after bleaching was measured indirectly and evaluated using this method. The tooth surface has a slight curvature that must be flat to measure the microhardness of the surface. In this experiment, the surface of the specimen was polished with 600-grit carborundum paper discs, which reduced the curvature of the surface and enabled constant measurements of the microhardness. Also, during the bleaching process and after remineralization, the specimens were stored in artificial saliva at 37℃ to reproduce the oral environment. Artificial saliva protects the demineralized surface from bleaching agents during bleaching and induces remineralization by interacting with the fluoride varnish or bioactive glass on the demineralized surface after bleaching (Andrew J, 2007).
In the previous studies, there has been controversy over the effect of whitening agents on the microhardness and morphological features of enamel surfaces according to differences in the method used, such as bleaching time and bleaching concentration. An in vitro study by Fatima N et al showed that bleaching with 16% CP agent resulted in insignificant effect on surface micro-hardness of enamel (Fatima N et al., 2016). On the other hand, teeth bleached with CP agent show calcium loss, deformed surface morphology, and reduced hardness and fracture resistance of the enamel (Tezel H et al, 2007; Al-Salehi SK et al, 2007; Jiang T et al, 2008). In situ and in vivo, a decrease in the microhardness of the surface of whitened enamel has been reported, which can be attributed to the loss of mineral content due to demineralization (Turkun M et al, 2002). In this study, the microhardness values of enamel surfaces showed a significant decrease after 14days for bleaching treatment, which supports the rejection of the first null hypothesis. Also, the specimens were immersed in artificial saliva. Amaechi and Higham suggested that the artificial saliva consisted of methyl-p-hydroxybenzoate 2g/liter, sodium carboxymethyl cellulose 10g/liter, KCl 8.38Mm, MgCl2·6H2O 0.29Mm, CaCl2·2H2O 1.13Mm, K2HPO4 4.62Mm, KH2PO4 2.4mM and fluoride 0.022 ppm (Rodrigues JA et al, 2001). The artificial saliva used in the study is similar to that previously proposed for artificial saliva. At 14 days after bleaching, the microhardness of the AS group increased, indicating that some remineralization may slowly occur due to the inorganic components in the artificial saliva. Indeed, saliva is supersaturated with respect to tooth minerals, and it plays an important role in remineralization by providing calcium and phosphoric acid to supplement the minerals lost in the demineralization process. In this experiment, the samples stored in artificial saliva showed a gradual increase in reduced microhardness after bleaching.
Fluoride can remineralize dental erosion lesions by increasing the resistance to acid attacks by forming a calcium fluoride layer that inhibits demineralization. The effects of post-bleaching fluoridation have also been confirmed (Lewinstein I et al, 2004; Bizhang M et al, 2006; Magalhães A et al, 2011). However, fluoride varnishes are removed by the action of the cheek, tongue, salivary flow, mastication, and oral hygiene procedures, thus exhibiting a short life span in the oral environment. Therefore, the varnish is liberated for a relatively short period of time before it disappears. The 5% NaF varnish used in this study was designed for the sustained release of fluoride, calcium, and phosphate. In this study, the fluoride varnish was applied to enamel surface for 4 hours because one study reported a reduction in concentration by more than 20% after about 4 hours of oral fluoride application (3M ESPE, 2012). In this experiment, the effects of the enamel surface on the ions released from the fluoride varnish were evaluated as a positive control. The FV group showed that the microhardness increased from (275.80±31.26) kg/mm2 to (320.53±20.18) kg/mm2 at 1 day after remineralization. This means that the contents of the lost minerals on the bleached enamel surface are provided by the fluoride varnish. Then, decreasing of microhardness was observed at 7 and 14 days after remineralization. This value was higher than that after bleaching, but there was no significant difference in the microhardness of the enamel before bleaching. This means indirectly that mineral deposition does not penetrate the depressed enamel prism cores or form a surface film by a chemical reaction with the teeth. Another cause is the replacement of artificial saliva every 24 hours, which also reduces the initial rich mineral environment by FV, rendering it unable to act as a fluoride varnish and reducing the microhardness.
The BAG group showed that the microhardness increased from (265.81±31.14) kg/mm2 to (318.81±23.51) kg/mm2 at 1 day after remineralization, was maintained, even after one week. Therefore, the second null hypotheses of the study were rejected. The initial microhardness of the enamel was increased rapidly; moreover, at 14 days after remineralization, it was higher than that of the artificial saliva group, but there was no significant difference. Although the microhardness was not significantly different from the AS group at 14 days after remineralization, remineralization of the BAG group occurred more rapidly than the AS group. This means rapid resistance of the enamel surface to the oral demineralization environment when BAG is applied. A recent study by Gjorgievska and Nicholson showed that the use of bioactive glass 45S5 (NovaMin), gold standard incorporated in toothpaste after bleaching, can remineralize the enamel surface by increasing the superficial Ca and P content (Gjorgievska E & Nicholson J.W, 2011). The bioactive glass 45S5 used in this study is an inorganic compound of highly biocompatible materials that reacts rapidly in the water environment and releases ions, such as Ca2+ and Na+ at a high rate (Gjorgievska E & Nicholson JW, 2010). When physiologically active glass or glass-ceramic particles come into contact with saliva or water, they release sodium, calcium, and phosphorus ions rapidly, which help remineralize the tooth surface (Burwell A.K et al, 2009). In this experiment, artificial saliva was used to reproduce the oral environment, which is expected to promote additional remineralization of BAG. The bioactive glass particles also adhere to the tooth surface and continue to release ions, resulting in the remineralization of the tooth surface after the initial application. These particles appeared to release ions in an in vitro study and transform to hydroxycarbonate apatite (HCA) for up to two weeks (Burwell A.K et al, 2009). This experiment also revealed the formative potential of hydroxycarbonate apatite on the remineralized enamel surface, resulting in increased microhardness. The application of BAG after bleaching is a good way to induce remineralization on the enamel surface, making the application of this BAG material a promising adjunct step to be used after bleaching therapy to avoid or minimize damage to the enamel surface.
In this study, we observed a decreased midrohardness in bleached enamel surfaces compared to untreated enamel surfaces. However, such change can be repaired with the use of remineralization agents such fluoride varnishes, bioactive glass powders and also artificial saliva which provide the ions necessary to remineralize these damaged surfaces.
CONCLUSION
Within the limitations of this study, the following conclusions were drawn:
- 1. Bleaching agents containing 15% carbamide peroxide reduce the enamel microhardness.
- 2. Remineralization treatments, such as bioactive glass or Fluoride varnish, rapidly increase the microhardness of the bleached enamel surface, but artificial saliva slowly increases the microhardness with no significant difference observed after 2 weeks.
- 3. Application of bioactive glass after bleaching formed surface deposits of remineralized material in the enamel.
References
- Al-Salehi, SK, Wood, DJ, Hatton, PV, (2007), The effect of 24h non-stop hydrogen peroxide concentration on bovine enamel and dentine mineral content and microhardness, J Dent, 35, p845-850.
-
Andersson, öH, Kangasniemi, I, (1991), Calcium phosphate formation at the surface of bioactive glass in vitro, J Biomed Mater Res, 25, p1019-1030.
[https://doi.org/10.1002/jbm.820250808]

- Andrew, J, (2007), Review of the effects of peroxide on enamel and dentine properties, J Dent, 35, p889-896.
- Basting, RT, Rodrigues, AL Jr, Serra, MC, (2003), The effects of seven carbamide peroxide bleaching agents on enamel microhardness over time, J Am Dent Assoc, 134, p1335-1342.
- Bizhang, M, Seemann, R, Duve, G, Romhild, G, Altenburger, JM, Jahn, KR, Zimmer, S, (2006), Demineralization effects of 2 bleaching procedures on enamel surfaces with and without post-treatment fluoride, Oper Dent, 31, p705-709.
- Burwell, A.K, Litkowski, L.J, Greenspan, D.C, (2009), Calcium sodium phosphosilicate (NovaMin): remineralization potential, Adv Dent Res, 21, p35-39.
- Burwell, A, Jennings, D, Greenspan, DC, (2010), NovaMin and dentin hypersensitivity in vitro evidence of efficacy, J Clin Dent, 21, p66-71.
- Chaudhary, A, Ingle, NA, Kaur, N, Gupta, R, (2013), Effect of Fluoridated Dentifrices on Microhardness of Enamel Surface: InVitro Study, J Adv Oral Res, 4, p11-16.
- Chuenarrom, C, Benjakul, P, Daosodsai, P, (2009), Effect of indentation load and time on knoop and vickers microhardness tests for enamel and dentin, Materials Res, 12, p473-476.
- Curtis, AR, West, NX, Su, B, (2010), Synthesis of nanobioglass and formation of apatite rods to occlude exposed dentine tubules and eliminate hypersensitivity, Acta Biomater, 6, p3740-3746.
- Ernst, CP, Marroquin, BB, Willershausen-Zonnchen, B, (1996), Effects of hydrogen peroxide–containing bleaching agents on the morphology of human enamel, Quintessence Int, 27, p53-56.
- Fatima, N, Abidi, SYA, Meo, AA, (2016), In Vitro Comparative Study of Two Different Bleaching Agents on Micro-hardness Dental Enamel, J Coll Physicians Surg Pak, 26, p83-86.
- Flaitz, CM, Hicks, MJ, (1996), Effects of carbamide peroxide whitening agents on enamel surfaces and caries-like lesion formation: A SEM and polarized light microscopic in vitro study, J Dent Child, 63, p249-256.
- Gjorgievska, E, Nicholson, JW, (2010), Enamel remineralization potential of two dentifrices based on CPP-ACP and Novamin (Calcium sodium-phosphosilicate), Acta Odontol Latinoam, 23, p234-239.
-
Gjorgievska, E, Nicholson, J.W, (2011), Prevention of enamel demineralization after tooth bleaching by bioactive glass incorporated into toothpaste, Aust Dent J, 56, p193-200.
[https://doi.org/10.1111/j.1834-7819.2011.01323.x]

- Haywood, VB, (1990), Nightguard vital bleaching: Current information and research, Esthetic Dentistry Update, 1, p20-25.
-
Haywood, VB, (1991), Overview and status of mouthguard bleaching, J Esthet Dent, 3, p157-161.
[https://doi.org/10.1111/j.1708-8240.1991.tb00991.x]

-
Hench, LL, Splinter, RJ, Allen, WC, Greenlee, TK, (1971), Bonding mechanisms at the interface of ceramic prosthetic materials, J Biomed Mater Res, 5, p117-141.
[https://doi.org/10.1002/jbm.820050611]

-
Jiang, T, Ma, X, Wang, Y, Tong, H, Shen, X, Hu, Y, Hu, J, (2008), Investigation of the effects of 30% hydrogen peroxide on human tooth enamel by Raman scattering and laser induced fluorescence, J Biomed Opt, 13, p14-19.
[https://doi.org/10.1117/1.2870114]

- Joiner, A, (2006), The bleaching of teeth: A review of the literature, J Dent, 4, p412-419.
- Josey, AL, Meyers, IA, Romaniuk, K, Symons, AL, (1996), The effect of a vital bleaching technique on enamel surface and the bonding of composite resin to enamel, J Oral Rehabil, 23, p244-250.
- Lewinstein, I, Fuhrer, N, Churaru, N, Cardash, H, (2004), Effect of different peroxide bleaching regimens and subsequent fluoridation on the hardness of human enamel and dentin, J Prosthet Dent, 92, p337-342.
- Magalhães, A, Wiegand, A, Rios, D, Buzalaf, MAR, Lussi, A, (2011), Fluoride in dental erosion, Monogr Oral Sci, 22, p158-170.
- Rodrigues, JA, Basting, RT, Serra, MC, Rodrigues Junior, AL, (2001), Effects of 10% carbamide peroxide bleaching materials on enamel microhardness, Am J Dent, 14, p67-71.
- Rodrigues, JA, Marchi, GM, Ambrosano, GM, Heymann, HO, Pimenta, LA, (2005), Microhardness evaluation of in situ vital bleaching on human dental enamel using a novel study design, Dent Mater, 21, p1059-1067.
-
Rodrigues, J.A, Oliveira, G.P.F, Amaral, C.M, (2007), Effect of thickener agents on dental enamel microhardness submitted to at-home bleaching, Braz. Oral Res, 21, p170-175.
[https://doi.org/10.1590/s1806-83242007000200013]

- Sa, Y, Sun, L, Wang, Z, Ma, X, Liang, S, Xing, W, Jiang, T, Wang, Y, (2013), Effects of two in-office bleaching agents with different pH on the structure of human enamel: An in situ and in vitro study, Oper Dent, 38, p100-110.
- Tezel, H, Ertaş, OS, Ozata, F, Dalgar, H, Korkut, ZO, (2007), Effect of bleaching agents on calcium loss from the enamel surface, Quintessence Int, 38, p339-347.
- Turkun, M, Sevgican, F, Pehlivan, Y, Aktener, BO, (2002), Effects of 10% carbamide peroxide on the enamel surface morphology: a scanning electron microscopy study, J Esthet Restor Dent, 14, p238-244.
-
Vollenweider, M, Brunner, TJ, Knecht, S, Grass, RN, Zenhnder, M, Imfeld, T, Stark, WJ, (2007), Remineralization of human dentin using ultrafine bioactive glass particles, Acta Biomater, 3, p936-943.
[https://doi.org/10.1016/j.actbio.2007.04.003]

- 3M ESPE, (2012), Vanish 5% Sodium Fluoride White Varnish with Tri-Calcium Phosphate-Technical Product Profile.