Bleaching of stained resin using nitrogen doped-TiO2 nanoparticles
Abstract
There has been increasing use of the H2O2-based teeth bleaching agents. The purpose of this study was to evaluate the bleaching effectiveness of the laser irradiation combined with nitrogen doped-TiO2 nanoparticles (NPs) on the stained resin. Nitrogen (N) doped-TiO2 NPs were prepared under sol-gel method. Light absorbance, X-ray diffraction patterns of NPs, and bleaching of methylene blue and stained resins were evaluated. For bleaching of stained resin, NPs-containing gel was used. For irradiation, light of two different wavelengths was used. Unlike TiO2, N-TiO2 showed high absorbance after 400 nm. N-TiO2, which have used TiN as a precursor, showed a new rutile phase at the TiN structure. For methylene blue solution, N-TiO2 with 3% H2O2 resulted in the greatest absorbance decrease after laser irradiation regardless of wavelength. For stained resin test, N-TiO2 with 3% H2O2 resulted in the greatest color difference after laser irradiation, followed by group that used N-TiO2 without 3% H2O2.
초록
H2O2가 치아미백에 많이 사용되고 있다. 본 연구는 레이저 광조사 하에서 질소가 도핑된 이산화티탄 (N-doped TiO2) 나노입자로 착색된 레진을 얼마나 미백시키는지를 평가한 것이다. 이를 위하여 시료를 졸-겔 방법으로 제조하고 광흡수도와 XRD를 평가하였다. 액상의 methylene blue와 분말입자를 함유한 젤을 이용하여 착색된 레진의 색변화를 측정하였다. 광조사를 위하여 파장이 다른 빛을 사용하였다. 그 결과 N-doped TiO2는 보통의 TiO2에 비하여 400 nm 이후에서 높은 흡수도를 보였고 N-doped TiO2는 TiN구조를 기반으로 새로운 루틸구조가 관찰되었다. 레이저가 조사되는 환경에서 methylene blue 용액과 착색된 레진은 3% H2O2를 함유한 N-TiO2 나노입자에 의해서 15% H2O2보다 더 큰 흡수도 감소와 색변화를 보였다.
Keywords:
Bleaching agent, N-doped TiO2, Absorbance, XRD, Color difference키워드:
미백제, N-doped TiO2, 흡수도, XRD, 색변화Introduction
Due to the high expectation for clean and white teeth regardless of gender and generation, there have been many trials from Roman era using urine to recent using peroxide-based agents. Thus far, to satisfy diverse needs of the users, many tooth bleaching agents, mainly peroxide-based, have been introduced and the resultant bleaching was mainly affected by the strength and treated time of agent regardless of products (1-3). To satisfy diverse needs of the users, many different bleaching agents are available now from OTC (over-the-counter) to specialist products.
There are many complicated causes of teeth discoloration, and they can be categorized into two factors: intrinsic and extrinsic (4-6). Of the factors, aging, pigments or metal ions in colored foods or drinks, and smoking are the prevalent sources of teeth discoloration. Generally colored compounds from these sources are adsorbed onto the tooth surface or absorbed into the subsurface and then present stain. Compounds adsorbed onto the tooth surface can be readily removed by routine tooth brushing. Abrasives in tooth paste easily detach the adsorbed compounds. However, stains by the absorbed compounds are not readily removed by tooth brushing if tooth is not fully wear out.
Bleaching (whitening) agents are the substance that makes the stained (discolored) teeth to be white. Also, they affect the color (shade) of resin composites (7, 8). Among them, peroxide-based bleaching agents (carbamide peroxide [CH6N2O3] or hydrogen peroxide [H2O2]) at the concentration of 3-3.5% or over to 30-35% are the common options. Bleaching by peroxide is achieved through the formation of reactive free radicals. Stable peroxide becomes radicals by external heat or ultraviolet light (9,10). Free radicals penetrate into the tooth subsurface and decompose stains of long molecular size into short size. During the bleaching process by radicals, mineral and water loss occurs and then dehydrated tooth with shortened stains gradually looks white (11, 12).
Despite excellent bleaching capability of peroxide-based agents, it usually takes some weeks to get acceptable whitening if used with lower concentration. Higher concentration is good for reducing treatment time (less than one hour with 2-3 treatments), however, higher concentration can make sharp poking pains on teeth and some loss of minerals and water from the treated teeth (13, 14). Both in user and practitioner sides, short treatment time and less teeth damage with acceptable whiteness of teeth would be highly welcomed.
Titanium dioxide (TiO2) induces photocatalytic reaction if interact with ultraviolet light (UV) (15-17). UV makes electrons in valence band transit to conduction band by leaving holes. Formed holes and moved electrons induce redox process and produce hydroxyl radicals. Formed radicals can effectively bleach stains in materials as oxygen-rich peroxide does. However, since UV induces carcinogenic problems, using in oral cavity is not recommended. To handle the problem, doped-titanium dioxide with gas or metal ions was suggested. According to diverse studies, doped-titanium dioxide showed high absorption of visible light and effective treatment of diverse bacteria and pollutants (15-17). The purpose of the present study was to evaluate if nitrogen (N)-doped TiO2 nanoparticles (NPs) can bleach stains under laser irradiation as H2O2 does. In this study, composite resin was used as a test material prior to teeth for a preliminary test.
Materials and Methods
1. Synthesis of N-doped TiO2 nanoparticles (NPs)
To synthesize N-doped TiO2 NPs, commercial TiN was used. At first, 62 mg TiN was added into 30 mL of distilled water while magnetic stirring, then 6.0 mL of HCl (35%) was dropwisely added into the above solution while continuously stirring for 30 min. The final concentration of HCl is 2.0 M. The solution was then transferred to a Teflon-lined autoclave and heated for 8 h at 180℃. After cooling down to room temperature, the precipitate was collected, washed with distilled water and absolute alcohol several times, and then dried in a 80℃ heat chamber for 24 h. All chemicals were analytical reagent grade, purchased from Sigma Aldrich (St. Louis, MO, USA), and used without additional purification.
2. Characterization and evaluation of NPs
Absorbance of NPs was measured using UV-VIS spectrophotometer (Jasco V-670, Jasco, Tokyo, Japan) by taking BaSO4 as reference.
X-ray diffraction (XRD) patterns of NPs were obtained using an Ultima IV multipurpose XRD system (Rigaku, The woodland, TX, USA) at the 2θ angle between 20 and 80 degrees with a scanning speed of 0.1°/min.
For stained composite resin test, a dental composite resin (P60, 3M ESPE, St. Paul, MN, USA) of shade A3 was chosen and made a 1 mm thick disc using a metal mold. Specimens were light cured using a light-curing unit for 40 s and aged for 24 h at 37℃ dry chamber. After 24 h, specimens were stored in a coffee-filled container for staining. For this, 1.6 g of coffee powder (Maxim Kanu, Dongsuh, Seoul, Korea) was mixed in 10 mL distilled water. Staining was continued for 1 week and the coffee was replaced every two days. After 1 week, carbomer gels were made by adding 1 g of carbomer 940 with 19 mL distilled water. Doped NPs was added in the gel to 1 wt% while stirring. After that, such made carbomer gel was pasted over the stained resin to 1-mm thick. Laser (405, 660 nm; LVI Technology Inc., Yongin, Korea; 50 mW/cm2) was irradiated on the pasted gel up to 3 h. Color of the stained composite resin before and after light irradiation (every 30 min) was measured using a spectrophotometer (CM3600d, Konica Minolta, Osaka, Japan) under the reflectance (%R) mode. To measure the color, the pasted gel over the resin was cleared and swiped using the wet cotton swab. The color difference was calculated as follows:
ΔE* = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2
where ΔL*, Δa*, and Δb* are the changes in L*, a*, and b* respectively. Here, L* represents the degree of grayness and corresponds to lightness. The parameter a* represents the red (for + a* value) - green (for – a* value) axis, whereas b* is a parameter in the blue (for – b* value) - yellow (for + b* value) axis. For this study, seven different test conditions were adopted: ① gel+distilled water (DW); ② Gel + 3% H2O2; ③ Gel+15% H2O2; ④ Gel+N-TiO2+405 nm laser; ⑤ Gel+N-TiO2+3% H2O2+405 nm laser; ⑥ Gel+N-TiO2+660 nm laser; ⑦ Gel+N-TiO2+3% H2O2+660 nm laser.
4. Statistical analysis
The result of color difference was analyzed by one-way ANOVA followed by a Tukey’s post-hoc test for multiple comparisons; p values <0.05 were considered significant.
Results
Figure 1 shows the tested powders for this study. TiN was used as a precursor for doping TiO2. Unlike white TiO2, N-TiO2 has dark brown color similar to its precursor TiN.
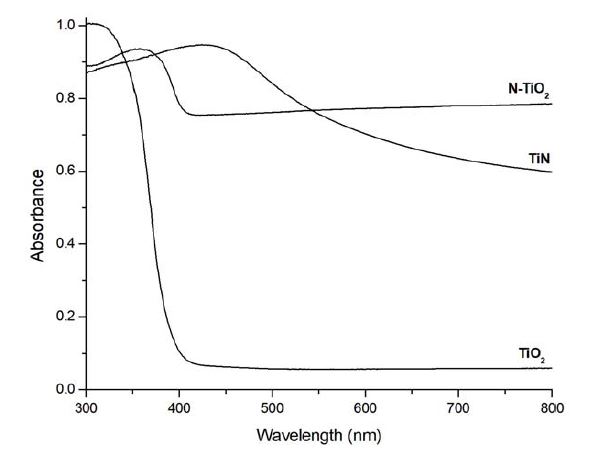
Figure 2 shows the absorbance spectrum of TiN, TiO2, N-TiO2. Unlike TiO2, doped N-TiO2 showed high absorbance after 400 nm.
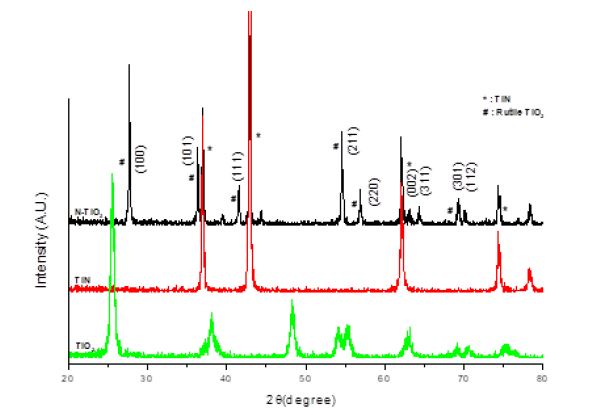
Figure 3 shows the XRD patterns of the tested samples. N-doped TiO2 had well-formed crystalline nature. Over the TiN peaks, the Bragg diffraction peaks indexed as (110), (101), (111), (211), (220), (002), (311), (301), and (112) are correspond to tetragonal rutile TiO2 (JCPDS, no. 21-1276).
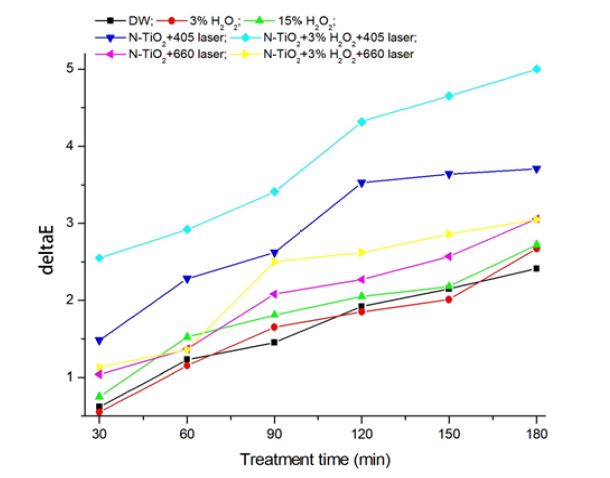
Figure 4 and Table 1 show the color difference (ΔE*) of stained resin by laser irradiation for 3 h (180 min). Initial color values (L*, a*, b*) of specimen resins before stain were 60.75±0.21, -0.85±0.07, 6.87±0.21, respectively. After stain for 1 week, these values changed to 49.34±0.76, 1.63±0.26, 14.33±0.55, respectively. For bleaching test, color values of the stained resin became the initial values before treatments. During laser irradiation, stained resins showed a linear color difference (R>0.94) regardless of incident light wavelength. Among the conditions, N-TiO2+3% H2O2+405 nm laser showed the greatest (up to 5.0) and significant (p<0.001) color difference (bleaching). Also, laser irradiated specimens showed higher color difference compared to that treated only with H2O2.
Discussion
Everybody wishes to have clean and white teeth. However, achieving and maintaining this wish is not easy owing to many daily obstacles and life styles. Tooth bleaching agents are materials that make this wish to be achieved easily and quickly with various effective dental treatments. However, peroxide (H2O2)-based bleaching agents usually confront two issues depending on the concentration of H2O2: long treatment time issue for low concentration agent; safety and oral damage issue for high concentration agent. Concerning teeth whitening, everybody wishes the bleaching to be achieved within short treatment time with minor soft tissue and teeth damage. The present study evaluated how N-doped TiO2 NPs affects bleaching (whitening) if they are irradiated with visible light.
The basic mechanism that explains bleaching (whitening) of stained material is cleavage of long molecular chain that causes discoloration into short molecular chain by reactive oxygen radicals (4,6). Optically short molecular chain becomes colorless. Peroxide-based bleaching agents are the source of radicals. To break H2O2 for forming radicals, one of two different energies are needed: heat or light. Heat breaks molecular bond of H2O2 which has weak O-O bond. Heat can boost radical formations, but it simultaneously produces unpleasant feeling on teeth and can cause pulp damage if temperature rises by heat over 5℃ for long time. UV light can break H2O2 because photon energy of UV exceeds the molecular binding energy of H2O2. However, owing to high photon energy, it can generate carcinogenetic damage on tissue. Hydrogen peroxide can also be broken down via photocatalytic effect of TiO2 with an aid of light. To initiate photocatalytic reaction from TiO2, at least 3.2 eV or over photon energy is required to make electrons in valence band to transit to conduction band by leaving holes in valence band. To supply this amount photon energy, UV light is required and in that case biohazard issue is inevitable.
Doping (gas or metal) ions into TiO2 as an impurity can lower photocatalytic activation energy from UV to visible light owing to narrowed energy band gap (18-20). One advantage using visible light as a light source is that it is free from biohazard issue if it is not operated at high power. In the present study, N-TiO2 has much higher absorbance than TiO2 after 400 nm (Figure 2). Compared to its precursor TiN, N-TiO2 does not have decreasing absorbance after 400 nm and this means that any other visible light can be absorbed in N-TiO2 NPs. For the present study, lasers of two different wavelengths have used: 405 and 660 nm with same light intensity.
Composite resins were immersed in coffee for 1 week to uniformly stain resin (21, 22). The stained resins have a coffee-adsorbed layer on their surface. To bleach the stained resins, carbomer gel which containing 1 wt% NPs without or with 3% H2O2 was pasted on the resin surface and then treated laser for 3 h (180 min). In the present study, as treated time increased, color difference by bleaching linearly increased. 405 and 660 nm laser treated specimens showed 1.36-1.87 and 1.12-1.15 times, respectively, higher color change (bleaching) compared to those treated only with 3 or 15% H2O2 after 3 h. In the laser treated specimens, addition of 3% H2O2 enhanced color change to 1.22-1.68 and 1.00-1.38 times, respectively, compared to those of no H2O2 specimens. The results here indicate that visible lasers (light) surely bleaching stain in conjunction with N-doped NPs, and in two lasers, shorter wavelength yields higher color difference (bleaching) than that by longer wavelength. Actually, from the relation E (photon energy)=hν=hc/λ, where h and λ are Planck’s constant and wavelength of the irradiated light, respectively, light of shorter wavelength is better than that of longer wavelength owing to higher photon energy of the light of shorter wavelength.
Conclusion
Within the limitations of the present study, the following conclusions could be reached:
1. N-TiO2 NPs showed the greatest color difference (ΔE*: up to 5.0) if combined with 3% H2O2 and 405 nm laser irradiation for 3 h. However, 15% H2O2 case showed only 2.7.
2. Two lasers (405 and 660 nm) achieved higher color difference compared to that by 15% H2O2. Between two lasers, 405 nm laser achieved higher bleaching (color difference) than that by 660 nm laser.
References
- Dourado Pinto AV, Carlos NR, Amaral FLBD, França FMG, Turssi CP, Basting RT. At-home, in-office and combined dental bleaching techniques using hydrogen peroxide: Randomized clinical trial evaluation of effectiveness, clinical parameters and enamel mineral content. Am J Dent. 2019;32(3):124-32.
-
Maran BM, Burey A, de Paris Matos T, Loguercio AD, Reis A. In-office dental bleaching with light vs. without light: A systematic review and meta-analysis. J Dent. 2018;70(1):1-13.
[https://doi.org/10.1016/j.jdent.2017.11.007]

-
Cura M, Fuentes MV, Ceballos L. Effect of low-concentration bleaching products on enamel bond strength at different elapsed times after bleaching treatment. Dent Mater J. 2015;34(2):203-10.
[https://doi.org/10.4012/dmj.2014-248]

-
Clifton M, Carey J. Tooth Whitening: What We Now Know. Evid Based Dent Pract. 2014;14(Supplement):70-6.
[https://doi.org/10.1016/j.jebdp.2014.02.006]

-
Joiner A, Luo W. Tooth colour and whiteness: A review. J Dent. 2017;67S:S3-S10.
[https://doi.org/10.1016/j.jdent.2017.09.006]

-
Kwon SR, Wertz PW. Review of the Mechanism of Tooth Whitening. J Esthet Restor Dent. 2015;27(5):240-57.
[https://doi.org/10.1111/jerd.12152]

-
de Andrade IC, Basting RT, Rodrigues JA, do Amaral FL, Turssi CP, França FM. Microhardness and color monitoring of nanofilled resin composite after bleaching and staining. Eur J Dent. 2014;8(2):160-5.
[https://doi.org/10.4103/1305-7456.130586]

- Park WK, Kang A, Son SA, Hur B, Kwon YH, Park JK. Effect of bleaching agents on the color change of different based composite resins. Kor J Dent Mater. 2013;40(2):121-7.
-
Buchalla W, Attin T. External bleaching therapy with activation by heat, light or laser-a systematic review. Dent Mater. 2007;23(5):586-96.
[https://doi.org/10.1016/j.dental.2006.03.018]

-
Yu H, Li Q, Wang YN, Cheng H. Effects of temperature and in-office bleaching agents on surface and subsurface properties of aesthetic restorative materials. J Dent. 2013;41(12):1290-6.
[https://doi.org/10.1016/j.jdent.2013.07.015]

-
Kim Y, Son HH, Yi K, Ahn JS, Chang J. Bleaching Effects on Color, Chemical, and Mechanical Properties of White Spot Lesions. Oper Dent. 2016;41(3):318-26.
[https://doi.org/10.2341/15-015-L]

-
de Araújo LS, dos Santos PH, Anchieta RB, Catelan A, Fraga Briso AL, Fraga Zaze AC, et al. Mineral loss and color change of enamel after bleaching and staining solutions combination. J Biomed Opt. 2013;18(10):108004.
[https://doi.org/10.1117/1.JBO.18.10.108004]

- de Oliveira SN, de Assunção IV, Borges BCD. Efficacy of ibuprofen and codeine + paracetamol to reduce immediate bleaching sensitivity caused by in-office tooth bleaching: A randomized, controlled, double-blind clinical trial. Am J Dent. 2018;31(4):195-8.
-
Pintado-Palomino K, Peitl Filho O, Zanotto ED, Tirapelli C. A clinical, randomized, controlled study on the use of desensitizing agents during tooth bleaching. J Dent. 2015;43(9):1099-105.
[https://doi.org/10.1016/j.jdent.2015.07.002]

-
Poulopoulos SG, Yerkinova A, Ulykbanova G, Inglezakis VJ. Photocatalytic treatment of organic pollutants in a synthetic wastewater using UV light and combinations of TiO2, H2O2 and Fe(III). PLoS One. 2019;14(5):e0216745.
[https://doi.org/10.1371/journal.pone.0216745]

-
Kazuya N, Akira F. TiO2 photocatalysis: Design and applications. J Photoch Photobio C. 2012;13(3):169-89.
[https://doi.org/10.1016/j.jphotochemrev.2012.06.001]

-
Etacheri V, Di Valentinc C, Schneider J, Detlef Bahnemannde D, Pillaifg SC. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J Photoch Photobio C. 2015;25:1-29.
[https://doi.org/10.1016/j.jphotochemrev.2015.08.003]

-
haozheng Hu, Fayun Li, and Zhiping Fan. Enhanced Visible Light Activity and Stability of TiO2 Nanopowder by co-doped with Mo and N. Bull Korean Chem Soc. 2012;33(4):1269-74.
[https://doi.org/10.5012/bkcs.2012.33.4.1269]

-
Gopinath K, Kumaraguru S, Bhakyaraj K, Thirumal S, Arumugam A. Eco-friendly synthesis of TiO2, Au and Pt doped TiO2 nanoparticles for dye sensitized solar cell applications and evaluation of toxicity. Superlattice Microst 2016;92:100e110.
[https://doi.org/10.1016/j.spmi.2016.02.012]

-
Ansari SA, MKhan MM, Ansaric MO, Cho MH. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016;40(4):3000-9.
[https://doi.org/10.1039/C5NJ03478G]

-
Reinhardt JW, Balbierz MM, Schultz CM, Simetich B, Beatty MW. Effect of Tooth-Whitening Procedures on Stained Composite Resins. Oper Dent. 2019;44(1):65-75.
[https://doi.org/10.2341/17-301-L]

-
Arocha MA, Mayoral JR, Lefever D, Mercade M, Basilio J, Roig M. Color stability of siloranes versus methacrylate-based composites after immersion in staining solutions. Clin Oral Investig. 2013;17(6):1481-7.
[https://doi.org/10.1007/s00784-012-0837-7]