Effect of dentin surface treatment and exclusive primer on bond strength of a self-adhesive resin cement
Abstract
The purpose of this study was to evaluate the effect of different dentin surface treatments on the bond strength of self-adhesive resin cement (G-CEM one). Human permanent molars were sectioned horizontally and standard smear layer was created. Teeth were divided into 4 groups according to the treatment methods: 1) no treatment, 2) 10% polyacrylic acid treatment, 3) exclusive primer (G-CEM one primer) treatment, 4) polyacrylic acid + exclusive primer treatment. After surface treatment, composite resin blocks were cemented with G-CEM one. After storage, specimens were cut into bars to measure the microtensile bond strength. Measured data were statistically analyzed. Confocal laser scanning microscopy was used to observe the bonding interface of cemented surface. Polyacrylic acid surface treatment group showed significantly lower μTBS than other groups (p<0.05). μTBS of exclusive primer treated groups was significantly higher than the control group (p<0.05). As a result, the exclusive primer improved dentin-cement bond strength. Polyacrylic acid treatment alone had negative effect on μTBS of G-CEM one and dentin.
초록
본 연구의 목적은 자가 접착 레진 시멘트(G-CEM one)의 접착 강도에 여러 상아질 표면 처리가 미치는 효과를 평가하는 것이다. 시편제작을 위해 인간 대구치를 수평으로 잘라 표준 도말층을 형성하였다. 상아질 처리 방법에 따라 처치를 시행하지 않은 대조군, 10% 폴리아크릴산을 처리한 군, 레진 시멘트의 전용 프라이머를 처리한 군, 폴리아크릴산 및 전용 프라이머를 처리한 군으로 시편을 분류하였다. 상아질 표면 처리를 시행한 후 치면에 미리 중합된 레진 블록을 G-CEM one 시멘트로 합착하였다. 시편을 물에 저장한 후 막대 시편을 제작하여 접착한 시편의 미세인장강도를 측정하였고 측정값은 통계 분석하였다. 또한 전용 프라이머를 적용한 시편의 상아질-시멘트접착 계면을 공초점 레이저 현미경으로 관찰하였다. 실험 결과 폴리아크릴산을 처리한 군의 접착 강도가 다른 군에 비해 유의하게 낮았고(p<0.05), 전용 프라이머를 처리한 군의 접착 강도는 대조군보다 유의하게 컸다(p<0.05). 결론적으로 시멘트 전용 프라이머는시멘트의 상아질 접착 강도를 유의하게 증가시켰고, 폴리아크릴산만 적용했을 경우 접착 강도에 부정적인 영향을 미쳤다.
Keywords:
Self-adhesive resin cements, Dentin surface treatment, Exclusive primer, Bond strength, Smear layer키워드:
자가 접착 레진 시멘트, 상아질 표면 처리, 전용 프라이머, 접착 강도, 도말층Introduction
Self-adhesive resin cements are widely used to lute indirect restorations fabricated using various materials in recent years. Manufacturers of self-adhesive cements suggest that surface treatment might not be necessary, which brings about the convenience of clinicians. The bond strength and bond stability between self-adhesive cements and dentin have been identified (1, 2). Selfadhesive cements do not require the etching procedure, but they have showed lower bond strength than conventional resin cements. Previously, many studies (3-9) have been doing research about dentin surface treatment before cementation to improve the bond strength of them.
Several studies (5, 6, 9) have reported that removal of smear layer helped the infiltration of monomers of self-adhesive cements and improves the bond strength. However, some kinds of self-adhesive cements showed no improvement or degeneration of bond strength. Mazzitelli et al. (7) evaluated the bond strength of three self-adhesive cements combined with dentin treatment using ethylene- diaminetetraacetic acid (EDTA) and polyacrylic acid to partially remove the smear layer. The bond strength of those self-adhesive cements had different tendency. Hydrophobic and solvent-free cement was unaffected, 2-hydroxyethyl methacrylate (HEMA)-based cement was affected negatively, and hydrophilic and water-containing cement was affected positively. Kambara et al. (4) also evaluated the relationship between self-adhesive cements and dentin surface treatments. Likewise, dentin surface treatments did not have a positive effect for all evaluated cements.
Smear layer could be removed by mild acidic agents like EDTA or polyacrylic acid. Polyacrylic acid is a mild acid that removes the smear layer partially but smear layer plugs in the tubules are not eliminated. Moreover, free calcium and phosphate ions on the dentin surface are released by removing the smear layers partially (9) EDTA also removes smear layer and smear plug, but do not increase in surface roughness unlike polyacrylic acid. (7)
Newly released self-adhesive cement (G-CEM one, GC Corp., Tokyo, Japan) includes its exclusive primer (G-CEM one primer, GC Corp., Tokyo, Japan) that has ability to provide “touch curing” of cements. When the cement and the primer are contacted, G-CEM one starts polymerizing immediately. Although it requires more steps to lute, but there are some benefits that consideration of film thickness is not necessary compared to the adhesives and the bond strength might be increased. This cement also includes functional monomer which might improve bond strength both to tooth and prosthetic materials. Manufacturer recommends application of the primer to improve the bond strength effectively (10).
The purpose of this study was to evaluate the effect of different dentin surface treatments on the microtensile bond strength (μTBS) of newly released self-adhesive resin cement.
Materials and Methods
Non-carious, non-treated human permanent molars were used after the approval of the Institutional Review Board of Pusan National University Dental Hospital (IRB, PNUDH-2018-021). The teeth were cleaned and stored in distilled water at 4℃. The roots of the teeth were embedded in self-cured acrylic resin (Tokuso Curefast, Tokuyama, Tokyo, Japan). Teeth were sectioned horizontally to the mid coronal level exposing flat dentin surface under water cooling. To create a standardized smear layer and flat surface, the dentin surface was polished with 600-grit silicon carbide abrasive paper for 60 seconds under running water with a polishing machine (Metaserv 250, Buehler, Lake Bluff, IL, USA) and rinsed with water for 30 seconds. Remained water was removed by absorbent paper.
Polymerized composite resin blocks were made by hybrid composite resin (Filtek Z-250, 3M ESPE, St. Paul, MN, USA) which incrementally layered by 2-mm into the silicone template (4 mm in thickness, 9 mm in diameter) and light cured for 40 seconds (BluePhase G2, Ivoclar Vivadent Inc., Amherst, NY, USA).
All teeth were randomly divided into 4 groups (n=10) according to the dentin surface treatment methods (Table 1): 1) no treatment (control), the composite resin blocks were cemented to the dentin surfaces without any dentin surface treatment, 2) polyacrylic acid treatment, dentin surface was treated with 10% polyacrylic acid (Dentin Conditioner, GC Corp., Tokyo, Japan) for 20 seconds, rinsed for 30 seconds with water and dried with absorbent paper, 3) exclusive primer treatment, exclusive primer (G-CEM one primer, GC Corp., Tokyo, Japan) was applied and scrubbed to the dentin surface for 10 seconds and blew a gentle stream of air for 5 seconds, 4) 10% polyacrylic acid and exclusive primer treatment, each treatment was consecutively applied.
After dentin surface treatment, polymerized composite resin blocks were cemented with self-adhesive resin cement (G-CEM one, GC Corp., Tokyo, Japan), followed by light-curing at 4 surfaces of the teeth for 10 seconds for each surface. The compositions of materials are described in Table 2. The cemented specimens were stored in distilled water at room temperature for 24 hours.
Stored teeth were sectioned vertically into 1×1×10 mm rods using a diamond saw (Accutom-50, Struers, Rø dovre, Denmark) with constant water cooling. The microtensile bond strengths (μTBS) of specimens of each group were measured using a universal testing machine (Bisco, Schaumburg, USA) at a crosshead speed of 1.0 mm/min until fracture. The maximum load was recorded in MPa. The bond failure modes were determined at a magnification 80x using a stereomicroscope (Global G6, Global Surgical Corporation, St. Louis, USA).
The microtensile bond strength data were analyzed using a one-way analysis of variance (ANOVA) and Tukey’s post hoc test at the 95% level of confidence. SPSS version 20 software (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis.
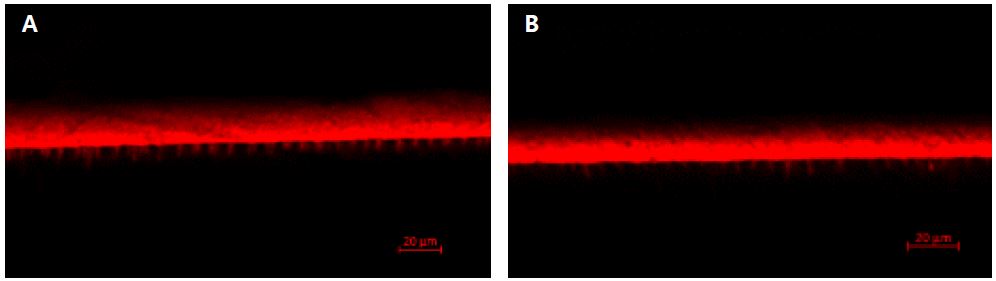
Confocal laser scanning microscopy (CLSM; TCS SL, Leica, Wetzlar, Germany) was used to observe the bonding interface of primed surface. As this self-adhesive cement could not infiltrate the dentin itself, only primer was dyed and applied for this study. Rhodamine B fluorescent dye (Daejung, Seoul, Republic of Korea) at a concentration of 0.01 wt% was added to the G-CEM one primer (11) and applied to the polished dentin surface. Two groups were divided according to whether they were treated with polyacrylic acid. Dentin surface preparation and polishing, application of polyacrylic acid and G-CEM one primer were performed by same protocol described above. G-CEM one cement was placed on the treated surface without composite resin block and light-cured for 40 seconds. The teeth were cut longitudinally into 0.7-mm thick slices and they were wet-polished with 600-grit SiC paper. The CLS microscope was used to obtain images of the bonded interfaces to observe penetration of the primer (11). The fluorescent images ware obtained with LSM-700 (Carl Zeiss, Oberkochen, Germany) in a 100-fold magnification and processed with ZEN 2.6 (blue edition) software (Carl Zeiss Microscopy GmbH, Jena, Germany).
Results
The microtensile bond strength data are described in Figure 1. Bond strength between dentin and self-adhesive cement was influenced by the surface treatment. Polyacrylic acid surface treatment group showed significantly lower μTBS than other groups (p<.05).
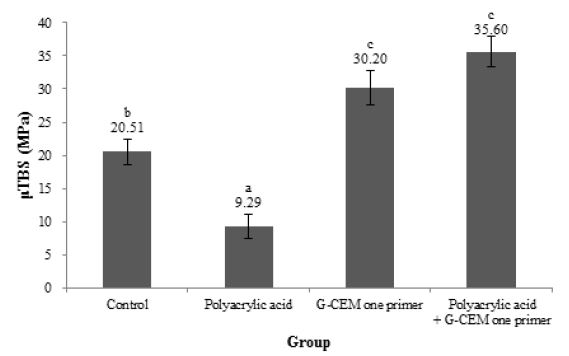
Comparison on mea n μTBS (MPa) of each dentin surface treatment group; Means with different letters are significantly different (P<.05).
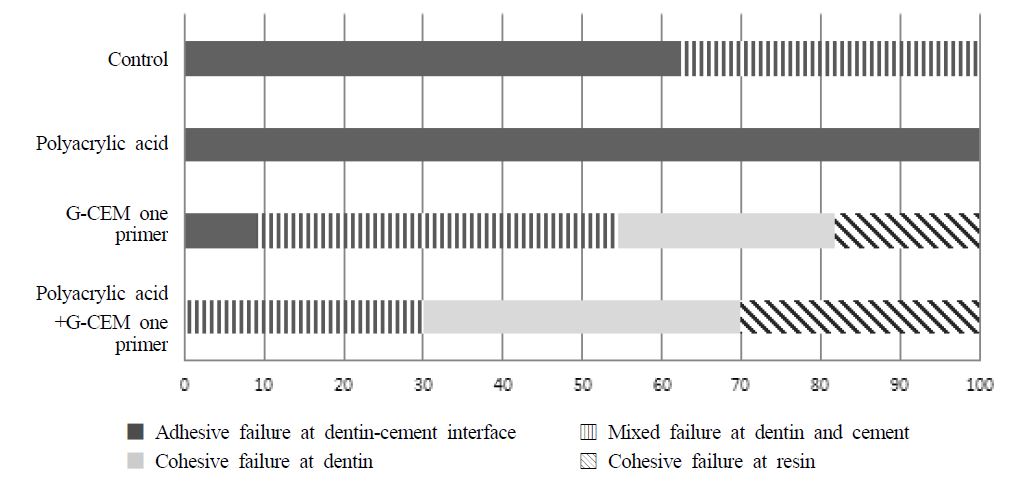
μTBS of exclusive primer group and polyacrylic acid and exclusive primer group was significantly higher than the control group (p<.05). μTBS of two exclusive primer-treated groups showed no significant differences regardless of polyacrylic acid surface treatment (p>.05). In terms of failure mode, for control group, adhesive failures were predominant, on the other hand, for polyacrylic acid treatment group, all specimens showed 100% adhesive failures between dentin and cement. Exclusive primer treatment and exclusive primer and polyacrylic acid treatment groups showed 45.4% and 70% cohesive failure, respectively. Especially, exclusive primer and polyacrylic acid treatment group showed no adhesive failure. Figure 3 presents CLSM images of G-CEM one primer interfaces. Both groups showed resin tag formation but their length and concentration have differences each other. In the polyacrylic acid treated group, more resin tags were made clearly.
Discussion
Self-adhesive cements are directly applied to the dentin without dentin conditioning. They use acidic monomers to demineralize the mineral tissue, however, they could not completely dissolve smear layer, and the interaction between dentin and cements is superficial (4). Insufficient dissolution of the smear layer could interrupt infiltration of monomers into the dentin, resulted in a formation of ‘weak’ link with the underlying dentin (10). Therefore, dentin surface treatment before cementation could give an opportunity to improve the monomer infiltration. In addition, an exclusive primer released with the cement might increase the wettability and reinforce the polymerization of cement. Under this perspectives, we investigated the microtensile bond strength of self-adhesive cement (G-CEM one) with different dentin surface treatments.
In this study, polyacrylic acid treatment on the dentin decreased bond strength of cement. However, the exclusive primer of the cement significantly increased bond strength between dentin and cement. When applying polyacylic acid followed by treating the primer, the bond strength did not be undermined. These results showed that removal of smear layer and use of exclusive primer influenced the bond strength of this cement.
For the polyacrylic acid treatment group, bond strength was significantly decreased compared to the control group. This result disagreed with other studies (5, 6, 9) which showed improved results. This might be caused by the monomer of self-adhesive cement. Acidic monomers in this self-adhesive cements demineralize and infiltrate into the dentin. (4) Generally, resin monomer infiltration is related to the applied concentration, viscosity of the solution, molecular weight or size, the affinity of monomers for the substrate and the time allowed for penetration. (12) When polyacrylic acid removes the smear layer and slightly demineralizes the underlying dentin, hydrated collagen fibril would be exposed. It is suggested that monomers in G-CEM one like UDMA have the hydrophobicity that it might less infiltrate to hydrated dentin even though UDMA has low molecular weight (13) which contribute to lower viscosity of cement.
For G-CEM one primer treatment group, bond strength was significantly higher than the control group. This primer has lower viscosity than the cement and its infiltration into the dentin is also better than the cement. Furthermore, since the primer makes hybrid layer by a hydrophilic monomer, the cement could contain hydrophobic materials. The hydrophobic behavior of the cement makes it limitedly soluble in water, then it also prevents substantial water uptake after curing. (13) Because excess water in the adhesive resin compromises the bond strength of adhesive, (13) restriction of water uptake might have an advantages to enhance the cement performance. In addition, according to the manufacturer, this exclusive primer provides “touch curing”, thus G-CEM one can be polymerized by with 3-way (light-, self-, and touch-curing). It seems that “touch curing” prevents the water absorption of the cement since this curing method promotes immediate polymerization.
For polyacrylic acid and G-CEM one primer treatment group, bond strength was significantly higher than the control group and was not significantly different from the primer treatment group. It means that G-CEM one primer enhances the bond strength regardless of presence of other dentin surface treatments. This primer includes 4-META, which has the hydrophilicity and enhances wetting (13) that might improve resin infiltration to dentin. Since hydrophilic monomer in this primer could infiltrate into the demineralized, hydrated collagen exposed dentin, G-CEM one primer improved the bond strength even on the surface treated dentin. Moreover, since polyacrylic acid would increase dentin surface roughness (6), polyacrylic acid and the primer application group showed higher bond strength than only the primer application group although not statistically significant. This result also explains the distribution of failure modes. Polyacrylic acid and the primer application group presents much cohesive failure than primer application only. CLSM image also support the results. G-CEM one primer formed resin tags in the dentinal tubules. This primer could penetrate the dentin covered by smear layer. Polyacrylic acid treated surface presented more resin tags than not treated one. It suggested that polyacrylic acid might enhance the resin monomer penetration. However, as more resin tags might not always guarantee the higher bond strength, it is hard to confirm that polyacrylic acid could imprave bond strength.
The results in this study are similar with other previous studies (4, 7). Kambara et al. (4) demonstrated the bond strength of three different cements after dentin surface treatment using EDTA. EDTA also could remove the smear layer on the dentin and slightly demineralize the dentin, which causes exposure of hydrated collagen fibril. In this previous study, the bond strength of Rely X Unicem (3M ESPE, St Paul, MN, USA) was not affected by EDTA treatment. Monomer in Rely X Unicem was assumed to have lower affinity than that of other cements because of its less infiltration into the dentin. And because of its high viscosity, this cement did not infiltrate into the dentin regardless of the opening of dentinal tubules. For the 4-MET/HEMA-containing cement (Breeze, Pentron Clinical Technologies, LLC, CT, USA) which was also used in the Kambara’s research, EDTA treatment significantly improved bond strength to dentin. Exposed, hydrated collagen causes moisture to be present on the dentin surface. 4-MET appears to be an important factor in polymerizing cement under hydrated dentin. Clearfil SA luting, which is HEMA-free but contains hydrophobic monomer like Bis-GMA and TEGDMA, showed decreased bond strength in the case of EDTA treatment. Finally, this study concluded that hydrophobic/hydrophilic properties of the cements significantly affected to the bond strength after smear layer treatment.
Mazzitelli et al. (7) also investigated whether pretreatments with EDTA or polyacrylic acid affects several self-adhesive cements. RelyX Unicem showed no increase in bond strength after EDTA and acrylic acid treatment because of its hydrophobic property and high viscosity that might interrupt resin infiltration. But chemical bonding area was also increased so that the bond strength was not quite changed. The bond strength of Bis-Cem (Bisco, Schaumburg, USA), HEMA-based cement, was significantly decreased. Although HEMA might have the potential to infiltrate into hydrated dentin, it is a water-soluble molecule that attracts water, leading to poor polymerization of cement. For G-CEM (GC, Tokyo, Japan), 4-META-based cement, polyacrylic acid treatment caused a significant increase in bond strength. This study also concluded that opening of dentinal tubules produces a water flow that might affect bond strength.
As a result, polyacrylic acid treatment affects the dentin bond strength of self-adhesive cement. This effect might depend on the types of monomers in the cement, and the clinicians should be careful to implement dentin conditioning according to the types of cements. For the exclusive primer, it achieved higher bonding performance in both dry and wet conditions than placing the cement alone. This result suggests that application of primer would prevent loss of bond strength in water contaminated dentin. Further studies are necessary to investigate the effectiveness of the primer in various dentin conditions.
Conclusion
Within the limitation of this study, exclusive primer for new self-adhesive resin cement improved dentincement bond strength significantly. Surface treatment with polyacrylic acid alone had negative effect on μTBS of G-CEM one self-adhesive cement and dentin. It is suggested that smear layer removal or slight demineralization do not always reinforce the performance of other cements. Clinicians should understand the properties of each cement before the cementation.
Acknowledgments
This study was supported by a 2-year Research Grant of Pusan National University.
References
-
Piwowarczyk A, Bender R, Ottl P, Lauer HC. Long-term bond between dual-polymerizing cementing agents and human bond dental tissue. Dent Mater 2007;23: 211–217.
[https://doi.org/10.1016/j.dental.2006.01.012]

-
Rodrigues RF, Ramos CM, Francisconi PA, Borges AF. The shear bond strength of self-adhesive resin cements to dentin and enamel: an in vitro study. J Prosthet Dent. 2015;113:220-7.
[https://doi.org/10.1016/j.prosdent.2014.08.008]

-
Bae IH, Son SA, Park JK. The effects of deproteinization and primer treatment on microtensile bond strength of self-adhesive resin cement to dentin. Kor JDent Mater. 2019;46:99-108.
[https://doi.org/10.14815/kjdm.2019.46.2.99]

-
Kambara K, Nakajima M, Hosaka K, Takahashi M, Thanatvarakorn O, Ichinose S, Foxton RM, Tagami J. Effect of smear layer treatment on dentin bond of self-adhesive cements. Dent Mater J. 2012;31:980-7.
[https://doi.org/10.4012/dmj.2012-031]

- Stona P, Borges GA, Montes MA, Júnior LH, Weber JB, Spohr AM. Effect of polyacrylic acid on the interface and bond strength of self-adhesive resin cements to dentin. J Adhes Dent. 2013;15:221-7.
-
Youm SH, Jung KH, Son SA, Kwon YH, Park JK. Effect of dentin pretreatment and curing mode on the microtensile bond strength of self-adhesive resin cements. J Adv Prosthodont. 2015;7:317-22.
[https://doi.org/10.4047/jap.2015.7.4.317]

-
Mazzitelli C, Monticelli F, Toledano M, Ferrari M, Osorio R. Dentin treatment effects on the bonding performance of self-adhesive resin cements. Eur J Oral Sci. 2010;118:80-6.
[https://doi.org/10.1111/j.1600-0722.2009.00703.x]

-
Pisani-Proença J, Erhardt MC, Amaral R, Valandro LF, Bottino MA, Del Castillo-Salmerón R. Influence of different surface conditioning protocols on microtensile bond strength of self-adhesive resin cements to dentin. J Prosthet Dent. 2011;105:227-35.
[https://doi.org/10.1016/S0022-3913(11)60037-1]

-
Broyles AC, Pavan S, Bedran-Russo AK. Effect of dentin surface modification on the microtensile bond strength of self-adhesive resin cements. J Prosthodont. 2013;22:59-62.
[https://doi.org/10.1111/j.1532-849X.2012.00890.x]

- G-CEM one Product Brochure 2018. GC.
-
Choi AN, Lee JH, Son SA, Jung KH, Kwon YH, Park JK. Effect of Dentin Wetness on the Bond Strength of Universal Adhesives. Materials (Basel). 2017:10;1224.
[https://doi.org/10.3390/ma10111224]

- Nakabayashi N, Pashley DH. Hybridization of dental hard tissues. Tokyo: Quintessence;1998. p.16-89.
-
Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757-85.
[https://doi.org/10.1016/j.biomaterials.2007.04.044]