3D architecture developments for spatial controls of periodontal ligament regeneration with angular orientations


Abstract
Various biomaterial-based, 3D architectures with micron-scaled geometries have been investigated for periodontal tissue neogenesis, and appropriate fabrication techniques for the scaffold manufacturing have been developed for pre-clinical or clinical situations. Periodontal tissues, the periodontal ligaments (PDLs), which are fibrous connective tissues between alveolar bone and cementum has been known as the major component that would generate biomechanical responses against mastication and occlusion. In particular, the angulations or orientations of PDL are critically important to transmit external stimuli and provide biomechanical adaptations for tooth-supporting functions. However, many studies still have demonstrated that optimal-biomechanical stimulations could re-make the configurations and orientations of PDL bundles with the low predictability in pre-clinical and clinical models. Here, we discussed the advanced technologies and geometric specifications for engineered PDL-guiding approaches spatiotemporally as 3D platforms.
초록
치주 복합 조직 재생을 유도하고 이들의 공간적 제어설계를 위한 생체재료 기반의 다양한 지지체들이 연구되고 있으며 이들은 전임상과 임상적 다양한 접근법들이 개발되고 있다. 경조직과 섬유 결합 조직들이 유기적으로 결합되어, 치아를 지지하는 구조체로서의 역할을 수행하는 치주 조직은 저작운동을 비롯한 다양한 외부의 기계적 자극을 수용하고 적절한 저항력을 생산하며 치아를 보호한다. 특히, 물리적/기계적 외부 자극으로부터의 생물학적/생리학적 반응들을 위해 치주 인대 섬유 조직의 특정 방향성은 중요한 요소 중 하나이다. 그럼에도 불구하고, 여전히 많은 연구자들은 치주 인대의 방향은 저작운동 등을 통한 자연 배향을 기대하고 있으며, 이들의 방향성을 제어하는 연구는 극히 제한적이며 도전적이다. 본 종설논문에서는 최근 발표된 치주 인대 방향성을 제어할 수 있는 구조 설계와 치주 인대의 배향에 필요한 입체 구조의 제작에 관한 내용을 보고한다.
Keywords:
Periodontal tissue, Periodontal ligament, Biomaterials, Regenerative medicine, Tissue engineering키워드:
치주 조직, 치주 인대, 생체재료, 재생의학, 조직공학1. Introduction
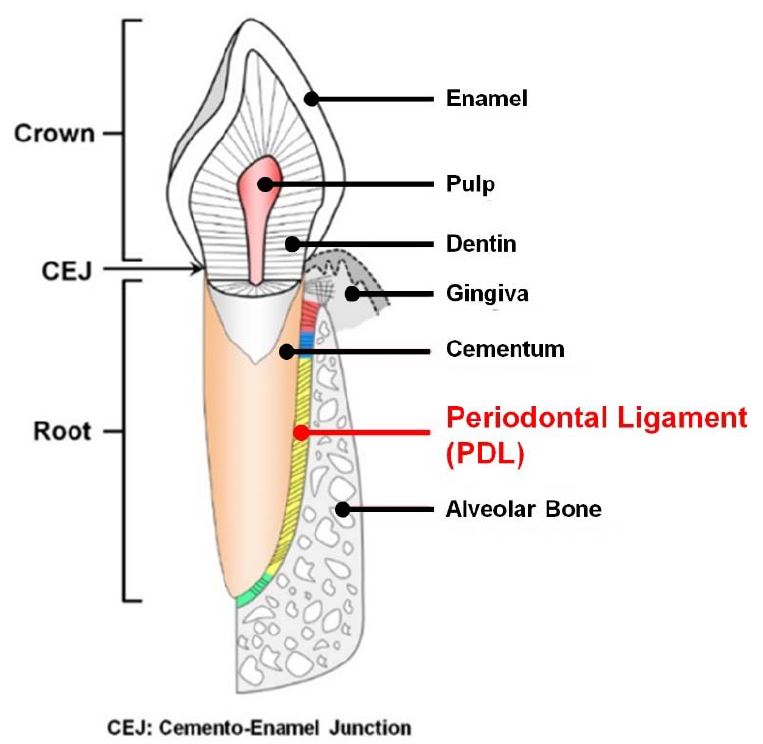
Periodontium or tooth-supporting complex includes four typical tissues with the structural compartmentalization and the anatomical hierarchy for systematic functions: gingiva, cementum (the mineralized layer on the root surface with 30~200 μm thickness), periodontal ligament (PDL; fibrous connective tissue in 200~300 μm thick space with specific angular orientations), and alveolar bone (mineralized tissue to support tooth and tooth-surrounding structures) (Figure 1) (1, 2). As the tooth-supporting structures, cementum, PDL, and alveolar bone are critically compartmentalized from the tooth-root surface with micron-scaled interfaces and their systemic integration could generate mechanical responses against mastication forces (3, 4). The cementum is deposited on the tooth dentin surface with similar mineral compositions to the bone tissues (5, 6) and classified with two different types on location such as acellular tissues for the attachment of PDLs at the coronal root surface and cellular tissues for periodontal repair by cementocyes within collagen matrices at the apical root surface (2, 7). The PDL is a specifically angulated, fibrous connective tissue and a collagen-based elastic fibrous bundle between two different mineralized tissues; cementum and alveolar bone (4, 8). According to location of tooth-root surfaces, PDLs could be categorized with specific orientations and Sharpey’s fibers, which are the terminal ends of principal fibers consisting of collagen bundles and contribute to insert ligamentous bundles ono the mineralized tissue surface for generation of mechanical responses and transmission of mastication and occlusion in order to protect teeth (1, 4, 9). Based on the advanced techniques, scaffolding system fabrications for the PDL tissue engineering were discussed with 3D architectures, which could control angular orientations of fibrous connective tissues, PDLs.
2. Characteristics of periodontal complex
Of the periodontal complex, the PDL has a well-organized, vascularity that provides a nutritive function for the periodontium (4). The alveolar bone is a dynamic tissue to generate appropriate responses to mechanical, microbiological, or biochemical influences by a remodeling process, which is associated with architectural changes and bone metabolic balances to adapt biomechanical environments (4, 10). In particular, the tooth socket structures in the alveolar bone have dense cortical plate which has the fibrous anchorage of the Sharpey’s fibers so, it is referred to as the bundle bone which can regulate physiological turnover of adjacent trabecular bone remodeling during orthodontic tooth movements or masticatory stimuli (6, 8). Moreover, numerous vascular channels in cribriform bone plates could be principal and the bone remodeling occurs to create the optimal trabecular structures with the balance between bone formation (by osteoblastic cells) and bone resorption (by osteoclastic cells) for communications between PDL bundles and the alveolar bone proper (11). That is, migrations of cycling mesenchymal progenitor cells could be activated in alveolar bone marrow to PDL interfaces for masticatory adaptations for the maintenance of hierarchical periodontal complexes. Fibrous connective tissue (PDL) bundles with specific angular organizations to the tooth-root surface serve to sense various biomechanical forces, to distribute transmitted masticatory loadings and to generate biomechanical responses (8, 12). In particular, the major compartments of four different PDL types are the obliquely or perpendicularly oriented PDL bundles and generally resist vertical and intrusive forces with the fiber anchorage between different mineralized layers; bone and cementum (4).
3. Periodontal disease
Periodontal disease is a highly prevalent inflammatory infectious disease and afflicts over 50% of the adult population in the United States, with severe disease concomitant with early tooth loss (13, 14). Moreover, 57.3% of the adult (55 < age < 65) suffers from periodontal disease and the prevalence of disease was exhibited greater including severe loss of periodontal attachments by the disease progression in Republic of Korea (15). Although initiated by microflora (or its metabolic products), it is now generally accepted that periodontal diseases result from a complex inflammatory and immunological response in susceptible hosts (16). Disease progression mainly leads to destruction of the individual periodontal complex like alveolar bone, PDL, and cementum as well as interconnected interfaces of alveolar bone-PDLs with subsequent tooth loss if left untreated (17-20). The recognition that periodontal regeneration can be achieved, including formation of new bone, new cementum and supportive PDL, has resulted in increased attempts to develop implantable dental biomaterials (20-22) as well as to understand cellular and molecular mechanisms for periodontal tissue reconstruction (1, 23). Additionally, the utilization of computer-assisted-design using medical images is beneficial because it can be used to generate spatially-compartmentalized scaffolds with anatomicallyspecific defect geometries, which are highly irregular at periodontal lesions (14, 24). Nevertheless, regenerative outcomes of existing therapies are still unpredictable and uncontrollable after regeneration treatments biologically and pathologically (1).
4. Periodontal tissue engineering and biomaterial-based scaffolding systems
The translational regenerative medicine and tissue engineering currently have multidisciplinary approaches for preclinical and clinical trials using engineering concepts such as scaffold constructs, stem cells, or bioactive molecules (25-29). In particular, stem cell therapies have strong potential to develop various clinical-therapeutic applications (25, 30, 31) and biochemical/biomolecular therapies using biologics such as growth factors, proteins, gene deliveries, or other bioactive molecules have been currently highlighted to accelerate tissue regeneration and defect healings (32-35). With long history, various growth factors like platelet-Derived Growth Factor (PDGF), bone morphogenetic protein (BMP), or fibroblast growth factor (FGF) have been typically investigated and widely developed for the stimulation of tissue formation and acceleration of target tissue regenerations with their optimal bioavailability and bioactivity (35-39). However, these approaches have still demonstrated difficulties to optimize the stem cell fate and control host tissue responses within unpredictable, physiologically complicated environments.
As paradigm shifts from the tissue replacement to the tissue regeneration, biomaterial-based scaffolds in the tissue engineering have been investigated to provide appropriate biologically-active and physiologically-adaptable microenvironments for tissue wound healing. For example of scaffolding system applications, localized and sustained delivery of growth factors is one approach with biodegradable scaffolds such as 1) calcium phosphate (CaP)-based hydroxyapatite material having osteoconductive characteristic to stimulate osteoblast cells for mineralized tissue formation (40-42) or 2) polymeric materials such as poly glycolic acid (PGA), poly lactic acid (PLA), or their copolymers (poly glycolic-co-lactic acid, PLGA) have also been utilized for hard or soft tissue regeneration in orthopedic/dental wound healing processes with interconnected structures (43-45). Using the biomaterials, appropriate 3D biological substitutes have been developed to restore, maintain, or improve tissue function (46-48). In particular, the porous scaffold or a synthetic extracellular matrix (ECM) plays a crucial role in defining the 3D geometry and providing microenvironments to promote various tissue regenerations or stem cell activations (49-53).
Many different types of scaffolding systems have been developed for multi-tissue and interface formations (54, 55), but to date, these approaches still have limitation for complete tissue integration, their functional restorations, and regenerated tissue-controllable architecture designs. To overcome limitations, computer design-based fabrication techniques using medical image data can allow the design of desired and predictable geometric structures with architectural compartmentalization for hierarchical constructs of multiple tissues in pre-clinical and clinical scenarios (56, 57). In particular, poly-ε-caprolactone-based (PCL) fiber-guiding scaffold with topographical specifications and spatial compartmentalization typically promoted regeneration of multiple tissues around tooth structures and guidance of specific PDL orientations (14, 24, 58). In addition, soft lithography was utilized to design sub-micron level topographic architecture in ECM or cell-substrate interaction studies of physical regulation of morphological changes of cells (59, 60). Moreover, physical-mechanical properties of ECM have demonstrated spatiotemporal directionalities of cell/tissue formation (61-63) and ECM influence on stem cell fate (30, 64).
5. Longitudinal pore architectures to control spatial PDL orientations in biomaterials
5.1. Freeze-casting method to create longitudinal pore architectures in gelatin scaffolds
The freeze-casting fabrication was utilized to generate angulated longitudinal pore structures in 3-D gelatin scaffolds to guide PDL (4). The fibrous connective PDL has four classifications like alveolar crest, horizontal, oblique, and apical fibers following existing regions and angular directions of PDL bundles. Using different freezing directions of 3-D gelatin constructs, the angulated directions of ice-crystal formations can be controlled and periodontal-mimic microenvironments could be created. Gelatin can be rapidly biodegraded and improve tissue ingrowth with spatiotemporal provision. In addition that gelatin scaffolds could improve cell adhesion or growth due to no chemical modifications, the directional freezing method is simple but predictably expected to angularly organize regenerated PDLs with structural similarities to native ligaments (4). Following the freeze-casting method, glutaraldehyde-crosslinked gelatin scaffolds were directionally frozen in paraffin molds in order to form directional pore architectures inside using the ice crystal growth kinetics, which could create the thermal gradients from the outer layer to inner core of gelatin scaffolds (4). According to the contact regions with dry ice, which could have the slower heat transfer rate than liquid nitrogen and gradually make the ice crystals, four different directionalities were defined such as apical, horizontal, horizontal and apical, and horizontal and coronal (4). After sublimation of the ice crystals by freeze-drying, the specific aligned pore structures were created with the angular similarity to the oblique PDL bundles around natural teeth and could be the 3D platform to guide PDL cells (4). However, the critical limitation of gelatin scaffolds including other natural polymer-based scaffolds are poor biomechanical properties. Due to the insufficiency to support tooth structures against various masticatory/occlusal loading conditions, the natural polymers are critically required to physically or chemically assemble with synthetic polymer constructs like PCL.
5.2. 3D stacking method to assemble electrospun nanofiber membranes with hydrogels
In addition, electrospinning fabrication technique was utilized to create highly aligned nanofiber membranes for periodontal tissue regeneration as the guided tissue regeneration (GTR) or guided bone regeneration (GBR) technique (65-68). Although the technique could provide various topological and morphological features to generate biological interactions between cell/tissue and material, the 2D nanofiber membranes still have the limitation for spatiotemporal PDL formations with structural similarities of natural PDLs like perpendicular or oblique orientations to tooth-root surfaces (69). Recently, z-directionally stacked electrospun 2D membranes were developed for directional controls of PDLs (66). After the synthetic polymer material (poly-ε-carpolactone and polyethylene glycol; PCE) was electrospun for nanofiber membranes with specific orientations, the membranes were stacked and assembled using the hydrogel material, the genipin-crosslinked chitosan (66). In in-vitro, aligned nanofiber membranebased scaffolds could control cell alignments following designed surface topologies with the predictability compared with the randomly organized nanofiber membrane group. Moreover, spatiotemporally organized fibrous connective tissue formations with perpendicular orientations to the tooth-root surfaces (66) and high expression levels of PDL-related genes, which could be presented tooth-supportive PDL tissues (66). The study significantly demonstrated that the 3D stacking method using electrospun nanofiber membranes with specific orientations facilitated to promote cell infiltration into porous architectures, cell/tissue orientations with unidirectional elongations, and extensive formation of mature collagen fibers (66).
6. Microgroove patterns to organize PDL cell/tissue angulations
6.1. Additive manufacturing to create microgroove patterns
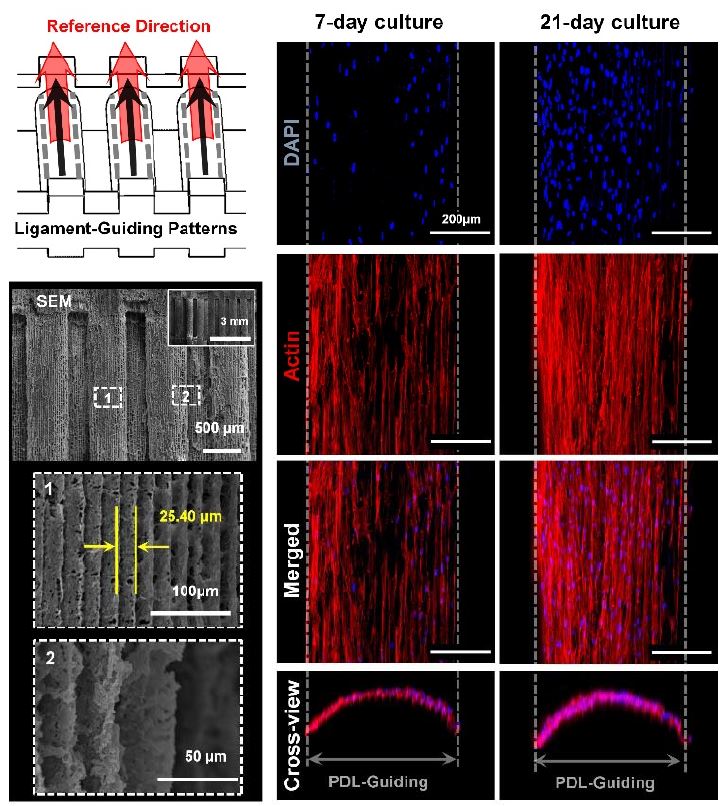
Various state-of-the-art approaches have been developed to organize cell alignments on two-dimensional (2D) substrates, which had topographical or morphological characteristics to generate various cell-material interactions (70-73). However, it is still limited to investigate precisely controllable method to deterministically create 3D printed architectures with cell-responsive micro-topographies, which are angulated microgroove patterns on spatial scaffolds (9, 14, 24, 58). The additive manufacturing system creates micron-scaled layer-bylayer artifacts, which are generally removed for smooth surface qualities. In particular, the created microgrooves (as known as stair-stepping errors or artifacts) were significantly considered for specific surface patterns, which can be programmed with the additive manufacturing layer-thickness (9). Recently, the image dataset of the beagle dog model was based to design the customized, defect-fit scaffolding system for the 1-wall periodontal defect (9). For periodontal complex neogeneses, the PDL-guiding architecture and the bone regeneration construct were designed individually and hybridized as a single system (Figure 3).
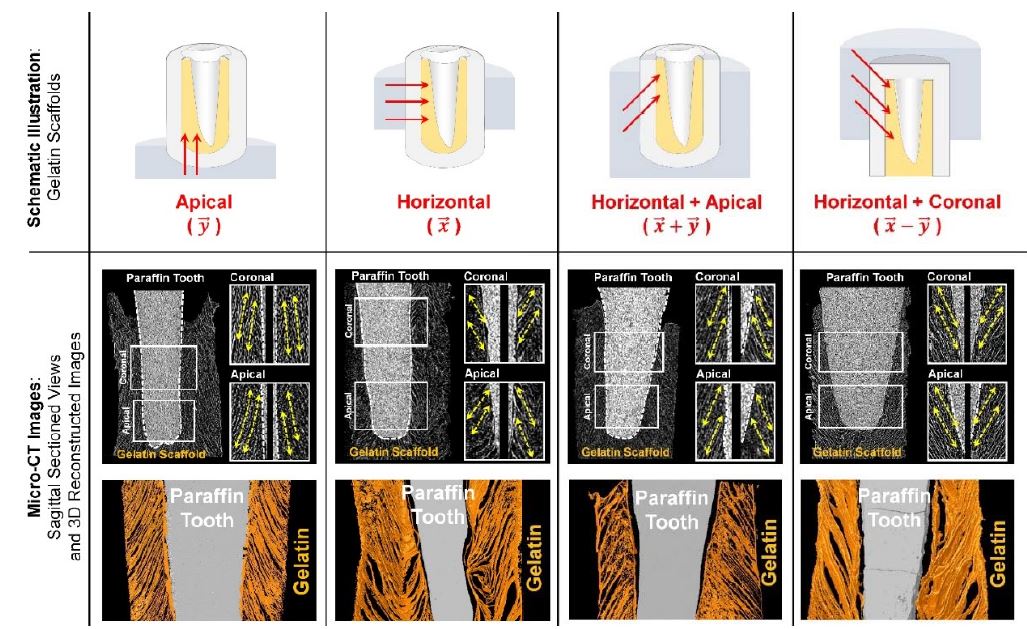
Longitudinal pore architectures in gelatin scaffolds. The freeze-casting method was utilized to create various angular organizations of pore structures in the biocompatible gelatin materials (4).
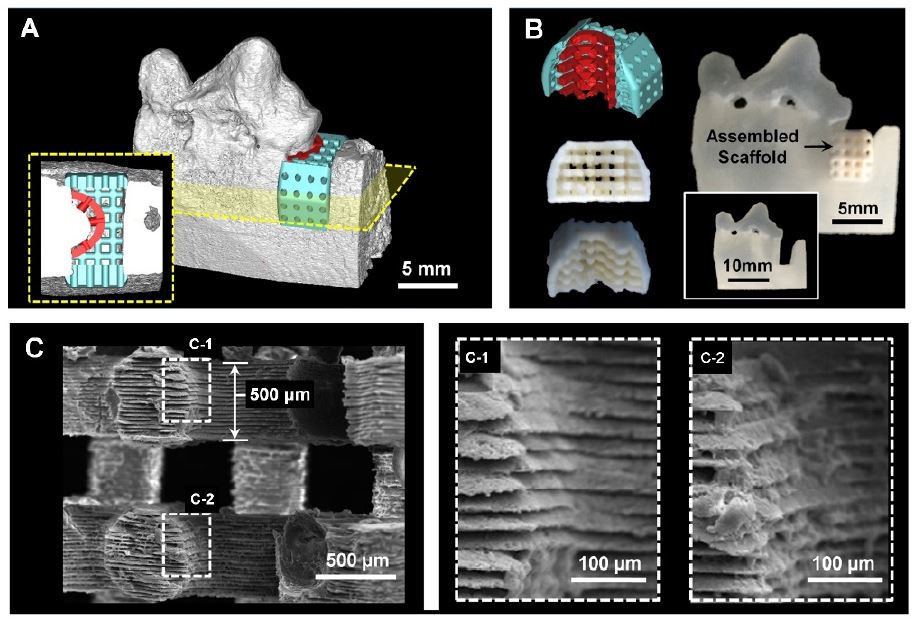
The scaffolding system for tissue-compartmentalized constructs using the 1-wall periodontal defect model of the canine. After design the scaffold based on the medical image dataset for periodontal ligament (PDL; red-colored) and bone (blue-colored) (A), the biopolymeric scaffold was manufactured by 3D printing technique (B). The scanning electron microscope (SEM) images showed the microgroove patterns on the PDL-guiding structures to organize PDL tissues, angularly (9).
The manufacturing strategy for biomimetic microenvironments can allow to integrate angulated microgroove patterns on ligament-guiding scaffolds and promote spatiotemporal directionalities and anisotropic organizations of fibrous connective cell/tissues (9, 14, 24, 58). Two in-vivo studies (mouse subcutaneous ectopic model (58) and rat periodontal fenestration defect model (14, 24)) had demonstrated that image-based scaffolding system to guide cells and tissues for periodontal complex neogenesis. In particular, as 3D engineered platform, fiber-guiding scaffolds successfully achieved fibrous connective tissues (PDLs) regeneration, orientation, and integration with the mineralized tissue layers with similar structures of natural periodontia (14, 24). Histology and immunofluoresence analysis of fibrous connective tissue formation and re-arrangement along the different topographic scaffolds showed that fiber-guiding topography potentiated and determined the guidance of fibrous tissue orientation against the dentin surface. In addition, periostin expression represented the functionalization of regenerated ligamentous tissues at the interface in fiber-guiding scaffold groups but, randomporous scaffold groups had significantly low expression levels (14, 24).
Recently, the different angulations of microgroove patterns on the PDL-guiding architectures by the additive manufacturing technique could predictably control human PDL cell orientations with parallel, oblique, or perpendicular directions to the designed PDL scaffold structures (9). Interestingly, optimal intervals of microgroove patterns were significant to regulate and organize highly populated or proliferated cells with specific angulations. Collectively, this study addressed that the angular patterns with optimal microgroove intervals (concretely, 25 μm-interval of microgroove patterns) can critically control directionalities of human PDL cells and tissues spatiotemporally using the 3D wax printing systems (Figure 4) (9).
6.2. Soft-lithographic strategy for spatial microgroove patterns on PDL architectures
In addition to the additive manufacturing technology, the lithographic strategy was investigated to create submicron-scaled microgroove patterns vertically for the perpendicular orientation of PDL cells and tissues (74, 75). In particular, Pilipchuk et al. investigated optimal topologies and patterns by the soft-lithographic technique in order to control the formations and orientations of PDL bundles in in-vitro and in-vivo (74, 75). There were two different in-vivo model systems for the study; 1) the ectopic subcutaneous transplantation study with assembled constructs (the scaffold and the human dentin slice) in a mouse model (75) and 2) the rat fenestration defect study to promote the regeneration of toothsupporting structures with PDL orientations (74). Although growth factors or any bioactive molecules could promote and accelerate tissue regenerations, the study significances clearly showed that topological approaches could angularly organize collagenous fibrous connective tissues (PDL-like tissue bundles) and facilitate to generate tooth-supportive constructs around the natural teeth (74).
7. Conclusion
The ultimate goal of periodontal tissue engineering is the regeneration of periodontal complexes such as gingiva, PDL, cementum and alveolar bone and tissue integrations for their functioning restoration as a tooth-supportive constructs. Although various strategies have been investigated for alveolar bone regeneration or cementum-like tissue deposition on the tooth-root surfaces, 3D orientations of engineered PDL bundles within submicron interfaces have been recently focused for specifically angular organizations of fibrous connective tissues using electrospinning, 3D printing system, soft-lithographic approaches, and freeze-casting method. Based on the geometric fabrication techniques to control micro-topologies and pore directionalities for PDL angulations, the spatiotemporal compartmentalization of multi-layered scaffolding systems should be developed and the engineered multiple tissue constructs should be validated under physiologically structural similarities.
Acknowledgments
This work was supported by National Research Foundation of Korea (NRF-2016R1D1A1B03935686).
Disclosure
The author has no conflict of interest for this work.
References
-
Park CH, Kim KH, Lee YM, Seol YJ. Advanced Engineering Strategies for Periodontal Complex Regeneration. Materials (Basel). 2016;9(1).
[https://doi.org/10.3390/ma9010057]

-
Menicanin D, Hynes K, Han J, Gronthos S, Bartold PM. Cementum and Periodontal Ligament Regeneration. Adv Exp Med Biol. 2015;881:207-36.
[https://doi.org/10.1007/978-3-319-22345-2_12]

-
Vaquette C, Saifzadeh S, Farag A, Hutmacher DW, Ivanovski S. Periodontal Tissue Engineering with a Multiphasic Construct and Cell Sheets. J Dent Res. 2019;98(6):673-81.
[https://doi.org/10.1177/0022034519837967]

-
Park CH, Kim KH, Rios HF, Lee YM, Giannobile WV, Seol YJ. Spatiotemporally controlled microchannels of periodontal mimic scaffolds. J Dent Res. 2014;93 (12):1304-12.
[https://doi.org/10.1177/0022034514550716]

-
Park CH, Oh JH, Jung HM, Choi Y, Rahman SU, Kim S, et al. Effects of the incorporation of epsilon-aminocaproic acid/chitosan particles to fibrin on cementoblast differentiation and cementum regeneration. Acta Biomater. 2017;61:134-43.
[https://doi.org/10.1016/j.actbio.2017.07.039]

-
Yamamoto T, Hasegawa T, Yamamoto T, Hongo H, Amizuka N. Histology of human cementum: Its structure, function, and development. Jpn Dent Sci Rev. 2016;52(3):63-74.
[https://doi.org/10.1016/j.jdsr.2016.04.002]

-
Foster BL. On the discovery of cementum. J Periodontal Res. 2017;52(4):666-85.
[https://doi.org/10.1111/jre.12444]

-
Kim JH, Park CH, Perez RA, Lee HY, Jang JH, Lee HH, et al. Advanced biomatrix designs for regenerative therapy of periodontal tissues. J Dent Res. 2014;93(12):1203-11.
[https://doi.org/10.1177/0022034514540682]

-
Park CH, Kim KH, Lee YM, Giannobile WV, Seol YJ. 3D Printed, Microgroove Pattern-Driven Generation of Oriented Ligamentous Architectures. Int J Mol Sci. 2017;18(9).
[https://doi.org/10.3390/ijms18091927]

-
Ho SP, Kurylo MP, Fong TK, Lee SS, Wagner HD, Ryder MI, et al. The biomechanical characteristics of the bone-periodontal ligament-cementum complex. Biomaterials. 2010;31(25):6635-46.
[https://doi.org/10.1016/j.biomaterials.2010.05.024]

-
Jiang N, Guo W, Chen M, Zheng Y, Zhou J, Kim SG, et al. Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement. Front Oral Biol. 2016;18:1-8.
[https://doi.org/10.1159/000351894]

-
Oshima M, Ogawa M, Tsuji T. Functional Tooth Regeneration. Methods Mol Biol. 2017;1597:97-116.
[https://doi.org/10.1007/978-1-4939-6949-4_8]

-
Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, Cdc Periodontal Disease Surveillance workgroup: James Beck GDRP. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914-20.
[https://doi.org/10.1177/0022034512457373]

-
Park CH, Rios HF, Jin Q, Sugai JV, Padial-Molina M, Taut AD, et al. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials. 2012;33(1):137-45.
[https://doi.org/10.1016/j.biomaterials.2011.09.057]

-
Woo DH, You HY, Kim MJ, Kim HN, Kim JB, Jeong SH. Risk Indicators of Periodontal Disease in Korean Adults. J Korean Acad Oral Health. 2013;37(2):95-102.
[https://doi.org/10.11149/jkaoh.2013.37.2.95]

-
Bartold PM, Van Dyke TE. Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol 2000. 2013;62(1):203-17.
[https://doi.org/10.1111/j.1600-0757.2012.00450.x]

-
H RR, Dhamecha D, Jagwani S, Rao M, Jadhav K, Shaikh S, et al. Local drug delivery systems in the management of periodontitis: A scientific review. J Control Release. 2019;307:393-409.
[https://doi.org/10.1016/j.jconrel.2019.06.038]

-
Liu J, Ruan J, Weir MD, Ren K, Schneider A, Wang P, et al. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells. 2019;8(6).
[https://doi.org/10.3390/cells8060537]

-
Chen FM, Jin Y. Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Eng Part B Rev. 2010;16(2):219-55.
[https://doi.org/10.1089/ten.teb.2009.0562]

-
Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809-20.
[https://doi.org/10.1016/S0140-6736(05)67728-8]

-
Vaquette C, Pilipchuk SP, Bartold PM, Hutmacher DW, Giannobile WV, Ivanovski S. Tissue Engineered Constructs for Periodontal Regeneration: Current Status and Future Perspectives. Adv Healthc Mater. 2018;7(21):e1800457.
[https://doi.org/10.1002/adhm.201800457]

-
Ivanovski S, Vaquette C, Gronthos S, Hutmacher DW, Bartold PM. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res. 2014;93(12):1212-21.
[https://doi.org/10.1177/0022034514544301]

-
Ramseier CA, Rasperini G, Batia S, Giannobile WV. Advanced reconstructive technologies for periodontal tissue repair. Periodontol 2000. 2012;59(1):185-202.
[https://doi.org/10.1111/j.1600-0757.2011.00432.x]

-
Park CH, Rios HF, Taut AD, Padial-Molina M, Flanagan CL, Pilipchuk SP, et al. Image-based, fiber guiding scaffolds: a platform for regenerating tissue interfaces. Tissue Eng Part C Methods. 2014;20(7):533-42.
[https://doi.org/10.1089/ten.tec.2013.0619]

-
Kaigler D, Pagni G, Park CH, Braun TM, Holman LA, Yi E, et al. Stem cell therapy for craniofacial bone regeneration: a randomized, controlled feasibility trial. Cell Transplant. 2013;22(5):767-77.
[https://doi.org/10.3727/096368912X652968]

-
Chaudhuri O, Mooney DJ. Stem-cell differentiation: Anchoring cell-fate cues. Nat Mater. 2012;11(7):568-9.
[https://doi.org/10.1038/nmat3366]

-
Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-41.
[https://doi.org/10.1016/j.addr.2012.04.008]

-
Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;32(36):9622-9.
[https://doi.org/10.1016/j.biomaterials.2011.09.009]

-
Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324(5935):1673-7.
[https://doi.org/10.1126/science.1171643]

-
Pitaru S, Tal H, Soldinger M, Noff M. Collagen membranes prevent apical migration of epithelium and support new connective tissue attachment during periodontal wound healing in dogs. J Periodontal Res. 1989;24(4):247-53.
[https://doi.org/10.1111/j.1600-0765.1989.tb01789.x]

-
Pagni G, Kaigler D, Rasperini G, Avila-Ortiz G, Bartel R, Giannobile WV. Bone repair cells for craniofacial regeneration. Adv Drug Deliv Rev. 2012;64(12):1310-9.
[https://doi.org/10.1016/j.addr.2012.03.005]

-
Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev. 2012;64(12):1292-309.
[https://doi.org/10.1016/j.addr.2012.01.016]

-
Ji W, Sun Y, Yang F, van den Beucken JJ, Fan M, Chen Z, et al. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm Res. 2011;28(6):1259-72.
[https://doi.org/10.1007/s11095-010-0320-6]

-
Kaigler D, Cirelli JA, Giannobile WV. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin Drug Deliv. 2006;3(5):647-62.
[https://doi.org/10.1517/17425247.3.5.647]

-
Suarez-Lopez Del Amo F, Monje A, Padial-Molina M, Tang Z, Wang HL. Biologic Agents for Periodontal Regeneration and Implant Site Development. Biomed Res Int. 2015;2015:957518.
[https://doi.org/10.1155/2015/957518]

-
Kitamura M, Nakashima K, Kowashi Y, Fujii T, Shimauchi H, Sasano T, et al. Periodontal tissue regeneration using fibroblast growth factor-2: randomized controlled phase II clinical trial. PLoS One. 2008;3(7):e2611.
[https://doi.org/10.1371/journal.pone.0002611]

-
Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9(4):519-26.
[https://doi.org/10.1016/j.ymthe.2004.01.016]

-
Anusaksathien O, Giannobile WV. Growth factor delivery to re-engineer periodontal tissues. Curr Pharm Biotechnol. 2002;3(2):129-39.
[https://doi.org/10.2174/1389201023378391]

-
Karadjian M, Essers C, Tsitlakidis S, Reible B, Moghaddam A, Boccaccini AR, et al. Biological Properties of Calcium Phosphate Bioactive Glass Composite Bone Substitutes: Current Experimental Evidence. Int J Mol Sci. 2019;20(2).
[https://doi.org/10.3390/ijms20020305]

-
Polo-Corrales L, Latorre-Esteves M, Ramirez-Vick JE. Scaffold design for bone regeneration. J Nanosci Nanotechnol. 2014;14(1):15-56.
[https://doi.org/10.1166/jnn.2014.9127]

-
Lu J, Yu H, Chen C. Biological properties of calcium phosphate biomaterials for bone repair: a review. RSC Adv. 2018;8:2015-33.
[https://doi.org/10.1039/C7RA11278E]

-
Li Y, Chu Z, Li X, Ding X, Guo M, Zhao H, et al. The effect of mechanical loads on the degradation of aliphatic biodegradable polyesters. Regen Biomater. 2017;4(3):179-90.
[https://doi.org/10.1093/rb/rbx009]

-
Gentile P, Chiono V, Carmagnola I, Hatton PV. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15(3):3640-59.
[https://doi.org/10.3390/ijms15033640]

-
Felix Lanao RP, Jonker AM, Wolke JG, Jansen JA, van Hest JC, Leeuwenburgh SC. Physicochemical properties and applications of poly(lactic-co-glycolic acid) for use in bone regeneration. Tissue Eng Part B Rev. 2013;19(4):380-90.
[https://doi.org/10.1089/ten.teb.2012.0443]

-
Chen FM, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86-168.
[https://doi.org/10.1016/j.progpolymsci.2015.02.004]

-
O'Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today 2011;14(3):88-95.
[https://doi.org/10.1016/S1369-7021(11)70058-X]

-
Eltom A, Zhong G, Muhammad A. Scaffold techniques and designs in tissue engineering functions and purposes: a review. Adv Mater Sci Eng. 2019;2019:1-13.
[https://doi.org/10.1155/2019/3429527]

-
Chen X, Fan H, Deng X, Wu L, Yi T, Gu L, et al. Scaffold Structural Microenvironmental Cues to Guide Tissue Regeneration in Bone Tissue Applications. Nanomaterials (Basel). 2018;8(11).
[https://doi.org/10.3390/nano8110960]

-
Gonzalez-Diaz EC, Varghese S. Hydrogels as Extracellular Matrix Analogs. Gels. 2016;2(3).
[https://doi.org/10.3390/gels2030020]

-
Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev. 2013;19(6):485-502.
[https://doi.org/10.1089/ten.teb.2012.0437]

-
Zhang H, Dai S, Bi J, Liu KK. Biomimetic three-dimensional microenvironment for controlling stem cell fate. Interface Focus. 2011;1(5):792-803.
[https://doi.org/10.1098/rsfs.2011.0035]

-
Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125(Pt 13):3015-24.
[https://doi.org/10.1242/jcs.079509]

-
Moffat KL, Sun WH, Pena PE, Chahine NO, Doty SB, Ateshian GA, et al. Characterization of the structure-function relationship at the ligament-to-bone interface. Proc Natl Acad Sci U S A. 2008;105(23):7947-52.
[https://doi.org/10.1073/pnas.0712150105]

-
Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J Biomed Mater Res A. 2008;86(1):1-12.
[https://doi.org/10.1002/jbm.a.32073]

-
Rasperini G, Pilipchuk SP, Flanagan CL, Park CH, Pagni G, Hollister SJ, et al. 3D-printed Bioresorbable Scaffold for Periodontal Repair. J Dent Res. 2015;94(9):153S-7S.
[https://doi.org/10.1177/0022034515588303]

-
Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4(7):518-24.
[https://doi.org/10.1038/nmat1421]

-
Park CH, Rios HF, Jin Q, Bland ME, Flanagan CL, Hollister SJ, et al. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials. 2010;31(23):5945-52.
[https://doi.org/10.1016/j.biomaterials.2010.04.027]

-
Sniadecki NJ, Desai RA, Ruiz SA, Chen CS. Nanotechnology for cell-substrate interactions. Ann Biomed Eng. 2006;34(1):59-74.
[https://doi.org/10.1007/s10439-005-9006-3]

-
Yim EK, Reano RM, Pang SW, Yee AF, Chen CS, Leong KW. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials. 2005;26(26):5405-13.
[https://doi.org/10.1016/j.biomaterials.2005.01.058]

-
Doyle AD, Yamada KM. Cell biology: Sensing tension. Nature. 2010;466(7303):192-3.
[https://doi.org/10.1038/466192a]

-
Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10(8):538-49.
[https://doi.org/10.1038/nrm2729]

-
Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol. 2009; 184(4):481-90.
[https://doi.org/10.1083/jcb.200810041]

-
Tenney RM, Discher DE. Stem cells, microenvironment mechanics, and growth factor activation. Curr Opin Cell Biol. 2009;21(5):630-5.
[https://doi.org/10.1016/j.ceb.2009.06.003]

-
Florjanski W, Orzeszek S, Olchowy A, Grychowska N, Wieckiewicz W, Malysa A, et al. Modifications of Polymeric Membranes Used in Guided Tissue and Bone Regeneration. Polymers (Basel). 2019;11(5).
[https://doi.org/10.3390/polym11050782]

-
Jiang W, Li L, Zhang D, Huang S, Jing Z, Wu Y, et al. Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015;25:240-52.
[https://doi.org/10.1016/j.actbio.2015.07.023]

-
Bottino MC, Thomas V, Schmidt G, Vohra YK, Chu TM, Kowolik MJ, et al. Recent advances in the development of GTR/GBR membranes for periodontal regeneration--a materials perspective. Dent Mater. 2012;28(7):703-21.
[https://doi.org/10.1016/j.dental.2012.04.022]

-
Shang S, Yang F, Cheng X, Walboomers XF, Jansen JA. The effect of electrospun fibre alignment on the behaviour of rat periodontal ligament cells. Eur Cell Mater. 2010;19:180-92.
[https://doi.org/10.22203/eCM.v019a18]

-
Yang M, Gao X, Shen Z, Shi X, Lin Z. Gelatin-assisted conglutination of aligned polycaprolactone nanofilms into a multilayered fibre-guiding scaffold for periodontal ligament regeneration. RSC Adv. 2019;9: 507-18.
[https://doi.org/10.1039/C8RA09073D]

-
Zheng L, Jiang J, Gui J, Zhang L, Liu X, Sun Y, et al. Influence of Micropatterning on Human Periodontal Ligament Cells' Behavior. Biophys J. 2018; 114(8):1988-2000.
[https://doi.org/10.1016/j.bpj.2018.02.041]

-
Kim JH, Ko SY, Lee JH, Kim DH, Yun JH. Evaluation of the periodontal regenerative properties of patterned human periodontal ligament stem cell sheets. J Periodontal Implant Sci. 2017;47(6):402-15.
[https://doi.org/10.5051/jpis.2017.47.6.402]

-
Qasim SB, Najeeb S, Delaine-Smith RM, Rawlinson A, Ur Rehman I. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent Mater. 2017;33(1):71-83.
[https://doi.org/10.1016/j.dental.2016.10.003]

-
Takahashi H, Nakayama M, Itoga K, Yamato M, Okano T. Micropatterned thermoresponsive polymer brush surfaces for fabricating cell sheets with well-controlled orientational structures. Biomacromolecules. 2011;12 (5):1414-8.
[https://doi.org/10.1021/bm2000956]

-
Pilipchuk SP, Fretwurst T, Yu N, Larsson L, Kavanagh NM, Asa'ad F, et al. Micropatterned Scaffolds with Immobilized Growth Factor Genes Regenerate Bone and Periodontal Ligament-Like Tissues. Adv Healthc Mater. 2018;7(22):e1800750.
[https://doi.org/10.1002/adhm.201800750]

-
Pilipchuk SP, Monje A, Jiao Y, Hao J, Kruger L, Flanagan CL, et al. Integration of 3D Printed and Micropatterned Polycaprolactone Scaffolds for Guidance of Oriented Collagenous Tissue Formation In Vivo. Adv Healthc Mater. 2016;5(6):676-87.
[https://doi.org/10.1002/adhm.201500758]