Effect of compositional variation of dental MTA cements on setting time
Abstract
The long setting time of dental MTA (Mineral Trioxide Aggregate) cement is a major disadvantage in clinical use. In this study, the setting time (ST) of nine commercial MTA cements was tested according to the ISO 6876:2012 standard (n = 5). Materials evaluated were ProRoot MTA (PR), Ortho MTA (Ortho), Retro MTA (Retro), Endocem MTA (Endocem), Endoseal MTA (Endoseal), One-Fil (OF), MTA Cem (MC), EZ-Seal (EZ), and Biodentine (BD). XRD and XRF analysis were performed to evaluate the effect of composition on ST. Kruskal-Wallis test as a non-parametric ANOVA followed by Duncan’s post hoc test was used for statistical analysis. The ST was PR > EZ > OF > Ortho > Retro > MC > Endoseal > BD > Endocem in decreasing order (p < 0.001). PR showed the longest (369.4 min) and Endocem showed the shortest (2.4 min) ST. Endocem (2.4 min), BD (16.0 min) and Endoseal (47.0 min) contained calcium carbonate. MC (48.8 min), Retro (43.6 min), Ortho (65.0), and OF (165.4 min), which had the next short setting time, contained dicalcium aluminate. In EZ (182.4 min), dicalcium silicate was the main crystalline phase rather than tricalcium silicate, which contributes to the early strength, and it contained calcium sulfate. Endocem, which showed the shortest setting time, showed the smallest d90 particle size of 6.12 μm. The information obtained from this study would be helpful in developing a composition for controlling the setting time of MTA cement and selecting a product with a setting time suitable for each clinical case.
초록
치과용 MTA (Mineral Trioxide Aggregate) 시멘트의 긴 경화시간은 임상사용에서 큰 단점이다. 본 연구는 시판되는 9종 MTA 제품의 경화시간을 ISO 6876:2012 표준방법에 따라 5회 반복 측정하였다. 평가된 재료는 ProRoot MTA (PR), Ortho MTA (Ortho), Retro MTA (Retro), Endocem MTA (Endocem), Endoseal MTA (Endoseal), One-Fil (OF), MTA Cem (MC), EZ-Seal (EZ), 및 Biodentine (BD) 이었다. 경화시간에 미치는 조성의 영향을 평가하기 위하여 XRD 및 XRF 분석을 시행하였다. 경화시간 결과값 사이의 유의성은 비모수 검정 방법인 Kruskal-Wallis 분석 후 Duncan 사후검정을 통해 다중분석하였다. 경화시간은 PR > EZ > OF > Ortho > Retro >MC > Endoseal > BD > Endocem 순 이었다 (p<0.001). PR은 가장 긴(369.4 분), Endocem은 가장 짧은(2.4 분) 경화시간을 보였다. Endocem (2.4 분), BD (16.0 분) 및 Endoseal (47.0 분)은 탄산칼슘(calcium carbonate)를 함유하였고, 그 다음으로 경화시간이 짧았던 MC (48.8 분), Retro (43.6 분), Ortho (65.0 분) 및 OF (165.4 분)는 알루민산 이석회(dicalcium aluminate)를 함유하였다. EZ (182.4 분)는 초기강도 형성에 기여하는 규산 삼석회(tricalcium silicate)보다는 규산 이석회(dicalcium silicate)가 주 결정상을 이뤘고, 황산칼슘(calcium sulfate)을 함유하였다. 가장 짧은 경화시간을 보인 Endocem은 가장 작은 d90 입도(6.12 µm)를 보였다. 본 연구결과는 MTA 시멘트의 경화시간 조절을 위한 조성 개발과 각 임상 증례에 적합한 경화시간을 갖는 제품의 선택에 도움이 될 것으로 사료된다.
Keywords:
MTA cement, Calcium silicate cement, Setting time, Gillmore needle, Composition키워드:
MTA 시멘트, 칼슘 실리케이트, 경화시간, 길모어 침, 조성Introduction
Calcium silicate cement (CS cement) is generally named as MTA (Mineral Trioxide Aggregate). Since the first report of the clinical use of Portland cement to root canal filling by a German dentist Dr. Witte (1). MTA was introduced to dentistry after a patent for a Portland cement-based endodontic material issued by Torabinejad and White was registered in 1995 and 1998 (2, 3). The major components of Portland cement are tricalcium silicate (C3S), dicalcium silicate (C2S), tricalcium aluminate (C3A), and calcium aluminoferrite (CAF). MTA is a dental cement formulated by adding radiopacifier such as bismuth oxide and zirconia oxide to Portland cement. In virtue of its excellent biocompatibility, sealing ability, and particularly excellent antibacterial and hard tissue forming ability (4-6), its clinical application fields are widening to pulp capping, pulpotomy, apexification, root perforations repair, intra/extra root resorption, orthograde root filling, retrograde root-end filling, and pulp vitalization (7, 8). However, its poor operability and too long setting time are significant drawbacks which limit its use in clinical cases (9, 10). Therefore, many studies are performed to improve these handling and sluggish setting properties, and to enhance the remineralization and hermetic sealing at the pulp-dentin interface (11, 12).
After mixing the MTA powder with water, tricalcium aluminate and calcium sulfate react with water to form ettringite, and tricalcium silicate and dicalcium silicate react with water to precipitate calcium silicate hydrate and calcium hydroxide crystals called portlandite, providing an initial high alkali environment (13). Tricalcium silicate hydrates faster than dicalcium silicate (14). In the in vitro biological solution, amorphous calcium phosphate is produced by electrostatic interaction between the calcium-rich calcium silicate hydrate (CSH) surface and HPO42- ions, which are converted over time to carbonate apatite found in bone, cartilage, enamel, and dentin (15).
However, MTA has several disadvantages such as high price, inconvenient operability, difficulty in removal during root canal retreatment, long setting time, and discoloration (12, 16-18). Among those, slow setting of MTA is the most significant disadvantage which hinder the satisfactory usage in clinical cases.
Many investigations were performed to shorten the setting time. For example, inclusion of hydration accelerators such as calcium chloride (CaCl2) (16, 19, 20), citric acid (21), calcium lactate gluconate solution (22), or sodium phosphate dibasic (23) in the composition were studied, There are various strategies in the MTA formulation like removal of calcium sulfate (24), addition of carboxylic ether polymer (superplasticizer) (25), use of gelatin (26) and chitosan oligosaccharide solution (27) in liquid form, or changing the proportions of C3S, C2S, calcium carbonate, and calcium aluminate in the material (28, 29). Particle size refinement (30) and delivering a photo- polymerization system using various organic resins (31) are another approaches to solve the long setting problem.
Comparing the setting times of MTA cements based on published reports needs careful consideration because test method details they adopt are frequently different between papers. By electronic searching the published articles relating to the setting time measurement, we could find that the experimental methods were quite inconsistent (9, 18, 20, 21, 31, 33-68). Even for the same MTA material, the reported setting time results were various among papers. This problem will make the clinicians difficult to objectively guess how the material will set and to select appropriate material for various clinical cases. In this study, the setting time of nine commercially available MTA cements were evaluated to provide information for correct selecting of MTA cement having an appropriate setting time in relation to various clinical cases.
The X-ray diffraction analysis (XRD), X-ray fluorescence spectroscopy (XRF), and particle size analysis were performed to investigate the effect of the compositional variations on the setting time of MTA.
Materials and Methods
1. Article search related to setting time measurement
An electronic published article search was conducted of the ScienceDirect (https://www.sciencedirect.com) site using keywords of MTA, mineral trioxide aggregate, and setting time for the papers published during 1995-2020, and thirty-seven papers that reported experimental descriptions and result values related to setting time were selected as analysis target papers among the eighty-five searched papers. In addition, for the MTA products included in this study that were not included in the papers searched via the above search method, four papers related to setting time were added to analysis target papers by additionally searching the Google Scholar Internet site, and a total of forty-one papers were selected and analyzed. Differences in the setting time results reported for each product, and the type of test standard, mold and test equipment used, and environmental test conditions applied were compared.
2. Setting time measurement
The setting time of nine commercially available MTA was evaluated according to ISO 6876:2012 method (32). The materials tested were ProRoot MTA (PR; Dentsply, USA), Ortho MTA (Ortho; BioMTA, Korea), Retro MTA (Retro; BioMTA, Korea), Endocem MTA (Endocem; Maruchi, Korea), Endoseal MTA (Endoseal; Maruchi, Korea), One-Fil (OF; Mediclus, Korea), MTA Cem (MC; Nexobio, Korea), EZ-Seal (EZ; Ezekiel, Korea), and Biodentine (BD; Septodont, France) (Table 1).
Gypsum molds having five holes (Diameter 10 mm, Height 1 mm) that can hold a sample were prepared under the procedure shown in Figure 1. Test materials were mixed at (23±2) ℃ and (50±5)% relative humidity according to the manufacturers’ instructions. The MTA mixture was filled in the preconditioned gypsum mold stored for 24 h maintained at (37±1) ℃ and not less than 95% relative humidity, and the surface was flattened using a slide glass, and the assemble was left in the incubator cabinet during setting and measurement. The setting time was measured using a Gillmore-type indenter having a mass of (100±0.5) g and a flat end of diameter (2±0.1) mm. When the setting time stated by the manufacturer approaches, the indenter needle was gently placed vertically on to the test material. The time elapsed from the start of mix until indentation ceased to be visible was recorded as a setting time (Figure 2). Five replicates were tested for each product. The significance between the setting time results of each product group was analyzed by the Kruskal-Wallis test as a non-parametric ANOVA followed by Duncan’s post-hoc test using SPSS 26.0 (Statistical Product and Service Solutions 26.0, IBM Co., Armonk, NY, USA).
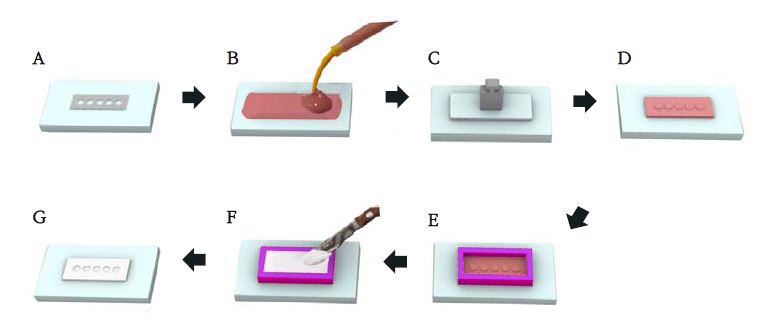
A) Metal mold (holes with 10 mm diameter and 1 mm depth; B) cover the metal mold with rubber impression material; C) cover the top with slide glass and weight; D) after setting of the rubber impression material, remove the metal mold to leave the negative form of the holes; E) box the periphery of the silicone mold with boxing wax leaving a space to be filled with secondary rubber impression material (after the setting of the rubber materials, remove the boxing wax); F) fill the boxed space with plaster (W/P ratio = 0.45); G) after the plaster set, remove the plaster mold from the rubber scaffold. The prepared plaster mold has the same shape as the metal mold with five sample holding holes.
3. X-ray diffractometric analysis
Crystalline phase analysis was performed for commercial MTA cement powder and for the MTA mixtures using X-ray diffractometer (XRD; EMPyrean, PANalytical, Netherlands). To hydrate MTA, it was mixed according to the manufacturer’s instructions, and the mixture was filled in a metal mold (Inner diameter 10 mm, Height 2 mm) and cured for 72 h in an oven at (37±1) ℃ and a relative humidity of 95% or more. The 72 h-aged specimen was dried for additional 72 h at 60 ℃, and then pulverized to a particle using agate mortar. The collected fine powder under 325 mesh was analyzed using XRD. The diffraction patterns were collected using a EMPyrean X-ray diffractometer (40 kV and 30 mA) with Cu Kα (λ = 1.54056 Å). The scanning speed was 1.8°/min and the scanning angle ranged from 2θ= 10-70°. The position and intensity of the diffraction peaks were analyzed using HighScore Plus (Panalytical, Netherlands) software with reference to the International Center for Diffraction Data (ICDD) to identify the crystalline materials.
4. X-ray fluorescence spectrometry analysis
An X-ray fluorescence spectrometry (XRF; Axios Minerals, Panalytical, Netherlands) was used for quantitative chemical analysis of the commercial MTA cements. The powder sample of about 5 g was mixed with a wax binder, and pressure molded to a circular pellet. We used Rh (rhodium) target and selected 3 kW (4 kW max.) for X-ray generator. The scan speed was 10° 2θ/s. Among the tested samples, Endoseal and OF were paste type and were calcined at 800 ℃ in an electric furnace in the air for 1 h for XRF analysis.
5. Particle size analysis
To analyze the particle size of the powder, a laser particle size analyzer (LS 13 320 XR, Beckman Coulter, Pasadena, CA, USA) was used. After dispersing in isopropyl alcohol for 30 s with an ultrasonic disperser, measurement was repeated three times by the DLS (dynamic laser light scattering) method. The particle size distribution was described using three reference points of d10, d50, and d90. The particle size value ‘d90’ means that 90% of the particles fall under this size. Measuring the particle size of Endoseal and OF was not appropriate because those were paste type.
Results
1. Article search results in relation to setting time measurement
By analyzing the forty-one papers (published during 1995-2020) relating to setting time of MTA cement, the reported setting times for the MTA cements included in this study were shown in Table 2. And, the detailed test conditions, such as applied standards, mold material type, environmental conditions of temperature and humidity, and used test equipment, were widely different as shown in Table 2.

Comparison of the reported setting times and test conditions used for the measurement of setting time of various commercial MTA cements
The applied standards were different as either ISO 6876, ISO 9917-1, ASTM C266, or ADA Spec #57 and the publication years of those applied standards were not consistent. And, there were even a number of papers that did not denote the referenced standard (18, 20, 33, 37, 38, 42, 44, 48, 55).
The mold materials used were different as metal, Teflon, acrylic, and dental gypsum, and metal molds were used most, followed by acrylic mold. The diameter and depth of the hole filled with the material were also inconsistent. Most of the measurement environmental conditions were 37 ℃ and 95% relative humidity, but there were quite a number of papers that did not specify them (9, 20, 31, 34, 36, 37, 39-41, 47, 48, 55, 57, 60, 62-64). The experimental instrument was either Gillmore needle or Vicat needle, and there was a difference in applied weight. Due to the difference in the selection of the referenced standard, some papers reported the initial and final setting times (in the papers to which either ASTM standard or ADA standard were applied) and the other papers reported the setting time (in the papers to which the ISO standard was applied).
2. Setting time
The setting time of the nine tested commercial MTA cements are shown in Table 3 and Figure 3. PR showed the longest setting time (369.4±13.8) min among tested materials. In next, EZ and OF showed setting time of 182.4 min and 165.4 min, respectively, and Ortho, MC, Endoseal, and Retro showed setting time of 65.0 min, 48.8 min, 47.0 min, and 43.6 min, respectively. BD showed the second fastest setting time (16.0 min), and Endocem had the shortest setting time (2.4 min).
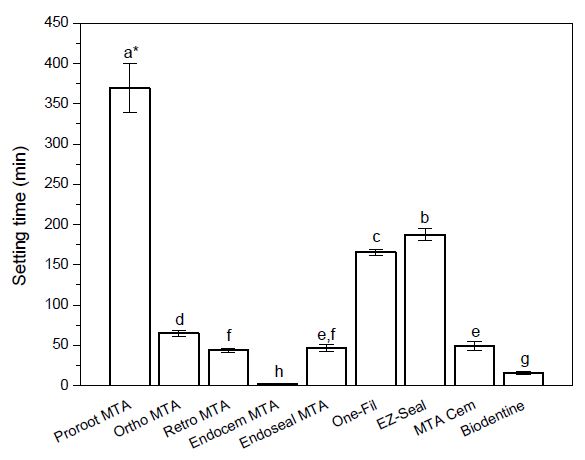
Mean and standard deviation of setting time of the tested materials (n = 5).*Materials with the same superscript letter are not statistically different in setting time at p = 0.05level by Duncan’s post-hoc test.
The setting time was shorter in the order of Endocem < BD < Retro < Endoseal < MC < Ortho < OF < EZ < PR.
3. X-ray diffractometric analysis
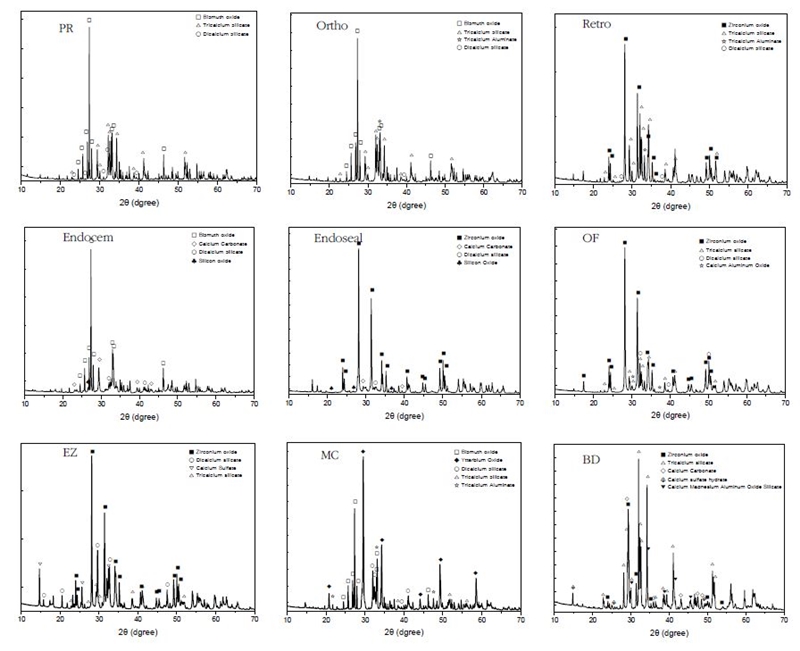
The XRD patterns of the nine commercial MTA cement powders and the hydrated samples aged for 3 d are shown in Figure 4, (A) and (B), respectively. In Figure 5, the crystal phase was assigned by searching the X-ray diffraction pattern of each MTA cement powder using the HighScore Plus software. In the XRD patterns of commercial MTA cements hydrated for 3 d, a crystal phase of calcium hydroxide (ICDD 00-044-1481) was observed with the diffraction peaks shown in the powder before hydration (Figure 4 (B)).
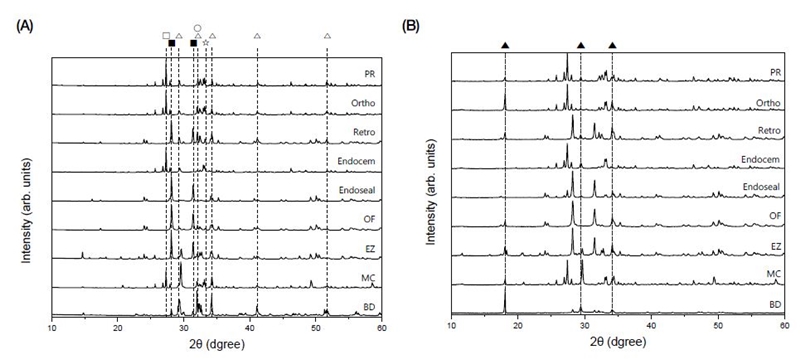
X-ray diffraction patterns of (A) non-hydrated MTA cement powder and (B) hydrated MTA cement set for 3 d; □, bismuth oxide (Bi2O3); ▇, zirconium oxide (ZrO2); △, tricalcium silicate oxide; ○, dicalcium silicate oxide; ☆, tricalcium aluminate; ▲, calcium hydroxide (Ca(OH)2).
Depending on the product, the XRD peaks of the radiopacifier such as bismuth oxide (Bi2O3), zirconium oxide (ZrO2), or ytterbium oxide (Yb2O3) were identified, and the peaks of tricalcium silicate (Ca3SiO5), dicalcium silicate (Ca2SiO4), and tricalcium aluminate (Ca3Al2O6) crystal phases were observed.
Even though the main peaks of tricalcium silicate are 34.4°, 22.2°, and 29.5° 2θ, it is difficult to distinguish them from overlapping peaks of dicalcium silicate. Thus, the crystalline phase was confirmed through rather weak peaks of tricalcium silicate appearing at around 22.8°, 30.0°, 38.5°, 46.8° 2θ (ICDD 00-042-0551). Even though the main peaks of dicalcium silicate appear near 33.4°, 32.5°, 32.6° 2θ, they mostly overlap with the tricalcium silicate peaks. Thus, the dicalcium silicate phase was confirmed by the presence of the rather low intensity peaks, which do not overlap with other crystal phase peaks, at around 23.0°, 31.0°, 39.5° 2θ(ICDD 00-033-0303).
The PR, Ortho, Endocem, and MC showed diffraction peaks at 27.3°, 33.2°, and 33.0° 2θ, indicating that they contained bismuth oxide (ICDD 00-014-0699) as a radiopacifier. Retro, Endoseal, OF, EZ and BD showed diffraction peaks at 28.1°, 31.4°, and 50.1° 2θ, demonstrating that they contained zirconium oxide (ICDD 01-072-1669). It was found that MC also contains ytterbium oxide (ICDD 01-088-2161) showing major diffraction peaks at 33.1°, 47.6°, and 59.2° 2θ in addition to bismuth oxide.
It was confirmed that, in addition to calcium silicate and radiopacifier, Endocem, Endoseal and BD contained calcium carbonate, and MC, Retro, Ortho and OF contained tricalcium aluminate. Calcium carbonate was confirmed by peak appearing at around 29.4°, 39.4°, 43.1° 2θ(ICDD 00-005-0586), and tricalcium aluminate was confirmed by diffraction peaks at 33.1°, 47.6°, 59.2° 2θ(ICDD 00-038-1429).
In EZ, dicalcium silicate was the main crystalline phase rather than tricalcium silicate, and it contained calcium sulfate to prevent flash set (Figure 5) (69).
4. X-ray fluorescence spectroscopy analysis
Table 4 shows the results of X-ray fluorescence spectroscopic analysis. The main constituent elements in all products were calcium and silicon, and bismuth oxide and zirconium oxide used as radiopacifier.

X-ray fluorescence analysis (XRF) results for the weight percentage of consisting elements (A) and estimated compounds (B) of the tested MTA cements(unit: wt.%)
PR, Ortho, Retro, and BD showed relatively high CaO content (over 54.01 wt.%) and SiO2 content (over 18.39 wt.%) compared to other products. The order of the product having high aluminum oxide content was Endocem, Ortho, Retro, MC, Endoseal, PR, OF, EZ, BD. EZ and BD had low aluminum oxide content of 0.16 and 0.12 wt.%, respectively.
The EZ, which had the second longest setting time, contained a high content of sulfur trioxide (SO3) at 5.31 wt.% and did not contain potassium oxide (K2O). Endocem, MC, PR, and Endoseal also showed 3.75-1.23 wt.% sulfur trioxide content in decreasing order. Retro and BD contained very small amounts, and Ortho and OF did not contain sulfur trioxide. Notably, the Endocem, which had the fastest setting time of 2.4 min, contained 3.75 wt.% of sulfur trioxide and 1.25 wt.% of potassium oxide (K2O).
Gray-colored Endocem and Endoseal contained 2.42 wt.% and 1.25 wt.% of iron oxide (Fe2O3), respectively. As a radiopacifier, the PR, Ortho, and Endocem contained only bismuth oxide, the Retro, Endoseal, OF, EZ, and BD contained only zirconium oxide, and the MC contained both bismuth oxide and ytterbium oxide in similar proportions. In addition, PR, Endocem, and Endoseal contained trace elements.
5. Particle size analysis
Table 5 shows the results of particle size analysis. The particle size of the tested materials was less than 8μm based on d50, but there was a considerable difference between tested products ranging from 70.81 μm (MC) to 6.12 μm (Endocem) when it is compared based on d90.
Discussion
In this study, the setting times of nine commercial MTA cements were evaluated to provide basic information for selecting the appropriate MTA product for each case in clinical practice. There are many reports evaluating the setting time, which is the biggest drawback of MTA cement.
Through searching and analyzing those research papers dealing with the setting time of MTA (9, 18, 20, 21, 31, 33-68), we could find that the setting time varies greatly depending on the published papers even for the same product. In Table 2, we compared the reported setting times and test conditions used for the measurement of setting time of various commercial MTA cements. Interestingly, it was found that the standards applied for the test, the mold material type, the experimental environment conditions, and the experimental equipment were inconsistent among reported papers. Moreover, considerably many published papers reported setting time results without stating the mold type used. Because there were many differences in the experimental methods among reported papers dealing with the setting time, it would be difficult for clinicians to select a suitable material based on objective information. Because many research papers did not mention the experimental environment, it was not able to determine whether the measurement was performed under an condition similar to oral environment (37 ℃, relative humidity of 95% or more).
Therefore, this study purposed to provide a guide in selecting a suitable product for each clinical situation by reporting the setting time results obtained by strictly observing the ISO test method that evaluates the setting time in an environment similar to the oral cavity (32). In ISO 6876:2012, for the case of hydraulic cement, it specifies to use a gypsum mold kept in moist 37 ℃ environment during the setting time measurement to simulate the condition that the cement material be provided with moisture like in oral cavity for the hydration process of the cement. But, there are few reports that evaluate accordingly. In this study, a gypsum mold was prepared (Figure 1) and the setting time was evaluated in compliance with the measurement method specified in the ISO standard. In particular, by using a modified anaerobic incubator having double doors, consistent evaluation was possible under constant temperature and humidity conditions similar to the oral environment (Figure 2). There was a significant difference in setting time depending on the product (p < 0.001) (Table 3, Figure 3).
In the XRD and XRF analysis (Figure 4, 5), there were differences in composition and major crystal phase depending on the product, and these differences were considered to affect the setting time of each product. In the XRD spectra of the commercial MTA, peaks of the radiopacifier such as bismuth oxide, zirconium oxide, or ytterbium oxide were identified, and the peaks of tricalcium silicate, dicalcium silicate, and tricalcium aluminate crystal phases similarly appearing in the ordinary Portland cement were observed. In addition to calcium silicate and radiopacifier, Endocem, Endoseal and BD contained calcium carbonate (ICDD 00-005-0586), and MC, Retro, Ortho and OF contained tricalcium aluminate (ICDD 00-038-1429). In EZ, dicalcium silicate was the main crystalline phase rather than tricalcium silicate which contributes to the early hydration reaction, and it contained calcium sulfate to prevent flash set (69). These two thing was considered as a possible contributing effect on the long setting time of EZ.
In the main composition of MTA, the order of hydration that occurs during the first few days is reported as tricalcium aluminate > tricalcium silicate > dicalcium silicate (14). Tricalcium aluminate hydrates rapidly and aluminate reacts violently with water if there is no gypsum present causing a flash set (69). The Ortho, Retro, MC, and OF contained tricalcium aluminate (Figure 5), by which could be an explanation why the setting time of those MTA cements was significantly faster than that of PR and EZ (p < 0.05). In PR, tricalcium silicate, which is responsible for early hydrate formation, was the main crystalline phase, and dicalcium silicate was contained in only a small amount. But, it did not contain calcium carbonate and tricalcium aluminate, by which would explain the longest setting time over 6 h. Tricalcium aluminate phase was not seen in MTA cements except Ortho, Retro, MC, and OF, and it has been reported that the absence or reduction of the aluminate phase has the advantage of improving workability (70).
Endocem, Endoseal and BD contained calcium carbonate (CaCO3) in their composition. Among the tested MTA cements, Endocem had the shortest setting time (2.4 min) followed by BD (16 min) (Table 3, Figure 4 and Figure 5). The addition of calcium carbonate contributes to significantly reducing the initial and final setting times, and improves the mechanical strength of cement paste in the initial stage of hardening (71).
Endocem having the shortest setting time (2.4 min) is reported to contain pozzolan particles (44). Pozzolans are siliceous or silicious and aluminous finely divided particles that do not react with cement by themselves, and react chemically with calcium hydroxide in the presence of water to form cementitious compounds (69). It is reported that pozzolans not only improve workability but also enhance long-term strength (72). Both Endocem and Endoseal products are reported by the manufacturer as being based on pozzolan cement, and silicon oxide peaks were confirmed in the XRD analysis of this study (Figures 4 and Figure 5). Endocem, which had the shortest setting time, had the smallest average particle size of 6.125 μm in d90. This small size of the particles is considered to contribute for the reduction of setting time (Table 3 and Table 5).
Based on the XRD analysis, the EZ, which had the second longest setting time, had dicalcium silicate as the main crystalline phase and contained calcium sulfate (Figure 5). As a result of XRF analysis, the EZ contained a high content of sulfur trioxide (SO3) at 5.31 wt.% and did not contain potassium oxide (K2O). Accordingly, it is judged that sulfur trioxide was mainly added in the form of calcium sulfate (CaSO4). This compositional characteristics is considered to contribute to the sufficient setting time (24). We think that EZ is more suitable to be used as a root canal sealing material rather than used for procedures requiring fast setting such as the cases of retrograde filling, pulp capping, and root perforation, etc. Endocem, which had the fastest setting time of 2.4 min, contained 3.75 wt.% of sulfur trioxide and 1.25 wt.% of potassium oxide (K2O) as shown in Table 4. It is conjectured that this components contribute to the early skeleton formation by the hydrate crystals resulting in fast setting (73).
As a radiopacifier, PR, Ortho, and Endocem contained only bismuth oxide, and Retro, Endoseal, OF, EZ, and BD contained only zirconium oxide. It is reported that bismuth oxide may cause tooth discoloration by MTA cement due to peroxidation when in contact with sodium hypochlorite solution (16). The MC contained both bismuth oxide and ytterbium oxide in similar proportions, and ytterbium oxide is reported to be an appropriate radiopacifier that does not affect physicochemical properties (74).
It is reported that particle size of a cement affects the setting time (75-77). The particle size of the tested MTA products did not show a big difference as less than 8 μm based on d50, but showed a big difference from 70.81 μm (MC) to 6.12 μm (Endocem) based on d90 (Table 5). It is considered that the particle size distribution affects the operability. Even if the average particle size is small, if relatively coarse particles are included in a certain amount, it will be disadvantageous because the handling characteristics would be similar to grains of sand. Endocem, which showed the shortest setting time, had the smallest d90 particle size (6.12 μm) among the evaluated products.
Conclusion
In the research reports evaluating setting time of MTA cement, the applied test method and obtained setting time values were quite different even for the same product, by which make the objective setting time information for the selection of appropriate material in clinical cases be difficult. The setting time results of the nine commercial MTA cements would be valuable information for the selection of suitable product having appropriate setting time for each clinical case. And, the information about the effects of the compositional variation on the setting time would be a good guideline for developing a MTA composition for controlling the setting time. In addition, in the future, it is required to verify the suitability of the standard test method stated in ISO 6876:2012 by comparing the setting time with the results obtained by applying other evaluation principles.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1A2C1009377).
References
- Witte DR. The filling of a root canal with Portland cement. J Cent Assoc Ger Dent. 1878;18:153-4.
- Torabinejad M, Linda L, White DJ, Dimas S. Tooth filling material and method of use. U.S. patent 5,415, 547,1995.
- Torabinejad M, Linda L, White DJ, Dimas S. Tooth filling material and method of use. U.S. patent 5,769, 638,1998.
-
Tanomaru-Filho M, Tanomaru JM, Barros DB, Watanabe E, Ito IY. In vitro antimicrobial activity of endodontic sealers, MTA-based cements and Portland cement. J Oral Sci. 2007;49(1):41-5.
[https://doi.org/10.2334/josnusd.49.41]

-
Vajrabhaya LO, Korsuwannawong S, Jantarat J, Korre S. Biocompatibility of furcal perforation repair material using cell culture technique: Ketac Molar versus ProRoot MTA. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(6):e48-50.
[https://doi.org/10.1016/j.tripleo.2006.05.015]

-
Apaydin ES, Shabahang S, Torabinejad M. Hard-tissue healing after application of fresh or set MTA as root-end-filling material. J Endod. 2004;30(1):21-4.
[https://doi.org/10.1097/00004770-200401000-00004]

-
Katsamakis S, Slot DE, Van der Sluis LW, Van der Weijden F. Histological responses of the periodontium to MTA: a systematic review. J Clin Periodontol. 2013;40(4):334-44.
[https://doi.org/10.1111/jcpe.12058]

-
Roberts HW, Toth JM, Berzins DW, Charlton DG. Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dent Mater. 2008;24(2):149-64.
[https://doi.org/10.1016/j.dental.2007.04.007]

-
Flores-Ledesma A, Barcelo Santana F, Bucio L, Arenas-Alatorre JA, Faraji M, Wintergerst AM. Bioactive materials improve some physical properties of a MTA-like cement. Mater Sci Eng C Mater Biol Appl. 2017;71:150-5.
[https://doi.org/10.1016/j.msec.2016.09.079]

-
Chng HK, Islam I, Yap AU, Tong YW, Koh ET. Properties of a new root-end filling material. J Endod. 2005;31(9):665-8.
[https://doi.org/10.1097/01.don.0000157993.89164.be]

-
Suhag K, Duhan J, Tewari S, Sangwan P. Success of direct pulp capping using mineral trioxide aggregate and calcium hydroxide in mature permanent molars with pulps exposed during carious tissue removal: 1-year follow-up. J Endod. 2019;45(7):840-7.
[https://doi.org/10.1016/j.joen.2019.02.025]

-
Yildirim T, Tasdemir T, Orucoglu H. The evaluation of the influence of using MTA in teeth with post indication on the apical sealing ability. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(3):471-4.
[https://doi.org/10.1016/j.tripleo.2009.04.036]

-
Camilleri J. The chemical composition of mineral trioxide aggregate. J Conserv Dent. 2008;11(4):141-3.
[https://doi.org/10.4103/0972-0707.48834]

-
Saghiri MA, Orangi J, Asatourian A, Gutmann JL, Garcia-Godoy F, Lotfi M, et al. Calcium silicate-based cements and functional impacts of various constituents. Dent Mater J. 2017;36(1):8-18.
[https://doi.org/10.4012/dmj.2015-425]

-
Niu LN, Jiao K, Wang TD, Zhang W, Camilleri J, Bergeron BE, et al. A review of the bioactivity of hydraulic calcium silicate cements. J Dent. 2014;42(5):517-33.
[https://doi.org/10.1016/j.jdent.2013.12.015]

-
Marconyak LJ, Jr., Kirkpatrick TC, Roberts HW, Roberts MD, Aparicio A, Himel VT, et al. A comparison of coronal tooth discoloration elicited by various endodontic reparative materials. J Endod. 2016;42(3):470-3.
[https://doi.org/10.1016/j.joen.2015.10.013]

- Ayatollahi F, Tabrizizadeh M, Hazeri Baqdad Abad M, Ayatollahi R, Zarebidoki F. Comparison of microleakage of MTA and CEM cement apical plugs in three different media. Iran Endod J. 2016;11(3):198-201.
-
Ber BS, Hatton JF, Stewart GP. Chemical modification of ProRoot MTA to improve handling characteristics and decrease setting time. J Endod. 2007;33(10):1231-4.
[https://doi.org/10.1016/j.joen.2007.06.012]

-
Tilakchand M, Pandey P, Shetty P, Naik B, Shetti S. The comparative evaluation of various additives on setting time and compressive strength of MTA Plus: An in vitro study. Endodontology. 2021;33(1):36-42.
[https://doi.org/10.4103/endo.endo_75_20]

-
Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006;32(6):569-72.
[https://doi.org/10.1016/j.joen.2005.08.006]

-
Lee BN, Hwang YC, Jang JH, Chang HS, Hwang IN, Yang SY, et al. Improvement of the properties of mineral trioxide aggregate by mixing with hydration accelerators. J Endod. 2011;37(10):1433-6.
[https://doi.org/10.1016/j.joen.2011.06.013]

-
Yang JC. The chemo-physical and biological properties of portland cement/bismuth oxide/zinc oxide composites hydrated by a novel calcium lactate gluconate (CLG) accelerant. Curr Nanosci. 2014;10(2):217-21.
[https://doi.org/10.2174/1573413709999131209123026]

- Ejaz M, Khan TA, Khan A, Amin N, Naseer A, Hussain S. Effect of sodium phosphate dibasic solution on the setting time of white mineral trioxide aggregate an in vitro study. JKCD. 2019;9(1):17-20.
-
Bramante CM, Kato MM, Assis GF, Duarte MA, Bernardineli N, Moraes IG, et al. Biocompatibility and setting time of CPM-MTA and white Portland cement clinker with or without calcium sulfate. J Appl Oral Sci. 2013;21(1):32-6.
[https://doi.org/10.1590/1678-7757201302200]

-
Wongkornchaowalit N, Lertchirakarn V. Setting time and flowability of accelerated Portland cement mixed with polycarboxylate superplasticizer. J Endod. 2011;37(3):387-9.
[https://doi.org/10.1016/j.joen.2010.11.039]

-
Wang CW, Chiang TY, Chang HC, Ding SJ. Physicochemical properties and osteogenic activity of radiopaque calcium silicate-gelatin cements. J Mater Sci Mater Med. 2014;25(9):2193-203.
[https://doi.org/10.1007/s10856-014-5258-5]

-
Kamali A, Javadpour S, Javid B, Kianvash Rad N, Naddaf Dezfuli S. Effects of chitosan and zirconia on setting time, mechanical strength, and bioactivity of calcium silicate-based cement. Int J Appl Ceram Technol. 2017;14(2):135-44.
[https://doi.org/10.1111/ijac.12636]

-
Lim J, Guk JG, Singh B, Hwang YC, Song SJ, Kim HS. Investigation on hydration process and biocompatibility of calcium silicate-based experimental Portland cements. J Korean Ceram Soc. 2019;56(4):403-11.
[https://doi.org/10.4191/kcers.2019.56.4.09]

-
Bernardi A, Bortoluzzi EA, Felippe WT, Felippe MC, Wan WS, Teixeira CS. Effects of the addition of nanoparticulate calcium carbonate on setting time, dimensional change, compressive strength, solubility and pH of MTA. Int Endod J. 2017;50(1):97-105.
[https://doi.org/10.1111/iej.12594]

-
Ha WN, Nicholson T, Kahler B, Walsh LJ. Methodologies for measuring the setting times of mineral trioxide aggregate and Portland cement products used in dentistry. Acta Biomater Odontol Scand. 2016;2(1):25-30.
[https://doi.org/10.3109/23337931.2015.1135746]

-
Gandolfi MG, Taddei P, Siboni F, Modena E, Ciapetti G, Prati C. Development of the foremost light-curable calcium-silicate MTA cement as root-end in oral surgery. Chemical-physical properties, bioactivity and biological behavior. Dent Mater. 2011;27(7):e134-57.
[https://doi.org/10.1016/j.dental.2011.03.011]

- International Organization for Standardization. ISO 6876:2012. Dentistry — Root canal sealing materials. Geneva: ISO; 2012.
-
Asgary S, Shahabi S, Jafarzadeh T, Amini S, Kheirieh S. The properties of a new endodontic material. J Endod. 2008;34(8):990-3.
[https://doi.org/10.1016/j.joen.2008.05.006]

-
Ding SJ, Kao CT, Shie MY, Hung C, Jr., Huang TH. The physical and cytological properties of white MTA mixed with Na2HPO4 as an accelerant. J Endod. 2008;34(6):748-51.
[https://doi.org/10.1016/j.joen.2008.02.041]

-
Hwang YC, Kim DH, Hwang IN, Song SJ, Park YJ, Koh JT, et al. Chemical constitution, physical properties, and biocompatibility of experimentally manufactured Portland cement. J Endod. 2011;37(1):58-62.
[https://doi.org/10.1016/j.joen.2010.09.004]

-
Lee SK, Lee SK, Lee SI, Park JH, Jang JH, Kim HW, et al. Effect of calcium phosphate cements on growth and odontoblastic differentiation in human dental pulp cells. J Endod. 2010;36(9):1537-42.
[https://doi.org/10.1016/j.joen.2010.04.027]

-
Hsieh SC, Teng NC, Lin YC, Lee PY, Ji DY, Chen CC, et al. A novel accelerator for improving the handling properties of dental filling materials. J Endod. 2009;35(9):1292-5.
[https://doi.org/10.1016/j.joen.2009.06.007]

-
Kao CT, Shie MY, Huang TH, Ding SJ. Properties of an accelerated mineral trioxide aggregate-like root-end filling material. J Endod. 2009;35(2):239-42.
[https://doi.org/10.1016/j.joen.2008.10.023]

-
Porter ML, Berto A, Primus CM, Watanabe I. Physical and chemical properties of new-generation endodontic materials. J Endod. 2010;36(3):524-8.
[https://doi.org/10.1016/j.joen.2009.11.012]

-
Kulan P, Karabiyik O, Kose GT, Kargul B. Biocompatibility of accelerated mineral trioxide aggregate on stem cells derived from human dental pulp. J Endod. 2016;42(2):276-9.
[https://doi.org/10.1016/j.joen.2015.10.015]

-
Islam I, Chng HK, Yap AU. Comparison of the physical and mechanical properties of MTA and portland cement. J Endod. 2006;32(3):193-7.
[https://doi.org/10.1016/j.joen.2005.10.043]

-
AlAnezi AZ, Zhu Q, Wang YH, Safavi KE, Jiang J. Effect of selected accelerants on setting time and biocompatibility of mineral trioxide aggregate (MTA). Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(1):122-7.
[https://doi.org/10.1016/j.tripleo.2010.07.013]

-
Chung H, Kim M, Ko H, Yang W. Evaluation of physical and biologic properties of the mixture of mineral trioxide aggregate and 4-META/MMA-TBB resin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(5):e6-11.
[https://doi.org/10.1016/j.tripleo.2011.04.005]

-
Kim M, Yang W, Kim H, Ko H. Comparison of the biological properties of ProRoot MTA, OrthoMTA, and Endocem MTA cements. J Endod. 2014;40(10):1649-53.
[https://doi.org/10.1016/j.joen.2014.04.013]

-
Che JL, Kim JH, Kim SM, Choi NK, Moon HJ, Hwang MJ, et al. Comparison of setting time, compressive strength, solubility, and pH of four kinds of MTA. Korean J Dent Mater. 2016;43(1):61-72.
[https://doi.org/10.14815/kjdm.2016.43.1.61]

-
Kaup M, Schafer E, Dammaschke T. An in vitro study of different material properties of Biodentine compared to ProRoot MTA. Head Face Med. 2015;11:16.
[https://doi.org/10.1186/s13005-015-0074-9]

-
Torabinejad M, Linda L, White DJ, Dimas S. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21(7):349-53.
[https://doi.org/10.1016/S0099-2399(06)80967-2]

-
Jang JH, Lee CO, Kim HJ, Kim SG, Lee SW, Kim SY. Enhancing effect of elastinlike polypeptide-based matrix on the physical properties of mineral trioxide aggregate. J Endod. 2018;44(11):1702-8.
[https://doi.org/10.1016/j.joen.2018.07.017]

-
Tu MG, Ho CC, Hsu TT, Huang TH, Lin MJ, Shie MY. Mineral trioxide aggregate with mussel-inspired surface nanolayers for stimulating odontogenic differentiation of dental pulp cells. J Endod. 2018;44(6):963-70.
[https://doi.org/10.1016/j.joen.2018.02.018]

-
Chiang YC, Chang HH, Wong CC, Wang YP, Wang YL, Huang WH, et al. Nanocrystalline calcium sulfate/hydroxyapatite biphasic compound as a TGF-beta1/VEGF reservoir for vital pulp therapy. Dent Mater. 2016;32(10):1197-208.
[https://doi.org/10.1016/j.dental.2016.06.013]

-
Akbari M, Zebarjad SM, Nategh B, Rouhani A. Effect of nano silica on setting time and physical properties of mineral trioxide aggregate. J Endod. 2013;39(11):1448-51.
[https://doi.org/10.1016/j.joen.2013.06.035]

-
Marciano MA, Camilleri J, Costa RM, Matsumoto MA, Guimaraes BM, Duarte MAH. Zinc oxide inhibits dental discoloration caused by white mineral trioxide aggregate angelus. J Endod. 2017;43(6):1001-7.
[https://doi.org/10.1016/j.joen.2017.01.029]

-
Cavenago BC, Del Carpio-Perochena AE, Ordinola-Zapata R, Estrela C, Garlet GP, Tanomaru-Filho M, et al. Effect of using different vehicles on the physicochemical, antimicrobial, and biological properties of white mineral trioxide aggregate. J Endod. 2017;43(5):779-86.
[https://doi.org/10.1016/j.joen.2016.12.023]

-
Moreno-Vargas YA, Luna-Arias JP, Flores-Flores JO, Orozco E, Bucio L. Hydration reactions and physico-chemical properties in a novel tricalcium-dicalcium silicate-based cement containing hydroxyapatite nanoparticles and calcite: A comparative study. Ceram Int. 2017;43(16):13290-8.
[https://doi.org/10.1016/j.ceramint.2017.07.027]

-
Abo El-Mal EO, Abu-Seida AM, El Ashry SH. A comparative study of the physicochemical properties of hesperidin, MTA-Angelus and calcium hydroxide as pulp capping materials. Saudi Dent J. 2019;31(2):219-27.
[https://doi.org/10.1016/j.sdentj.2018.09.004]

-
Marciano MA, Guimaraes BM, Amoroso-Silva P, Camilleri J, Hungaro Duarte MA. Physical and chemical properties and subcutaneous implantation of mineral trioxide aggregate mixed with propylene glycol. J Endod. 2016;42(3):474-9.
[https://doi.org/10.1016/j.joen.2015.10.014]

-
Massi S, Tanomaru-Filho M, Silva GF, Duarte MA, Grizzo LT, Buzalaf MA, et al. pH, calcium ion release, and setting time of an experimental mineral trioxide aggregate-based root canal sealer. J Endod. 2011;37(6):844-6.
[https://doi.org/10.1016/j.joen.2011.02.033]

-
Bortoluzzi EA, Broon NJ, Bramante CM, Felippe WT, Tanomaru Filho M, Esberard RM. The influence of calcium chloride on the setting time, solubility, disintegration, and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. J Endod. 2009;35(4):550-4.
[https://doi.org/10.1016/j.joen.2008.12.018]

-
Linhares Gda S, Cenci MS, Knabach CB, Oliz CM, Vieira MA, Ribeiro AS, et al. Evaluation of pH and calcium ion release of a dual-cure bisphenol A ethoxylate dimethacrylate/mineral trioxide aggregate-based root-end filling material. J Endod. 2013;39(12):1603-6.
[https://doi.org/10.1016/j.joen.2013.08.013]

-
Vivan RR, Zapata RO, Zeferino MA, Bramante CM, Bernardineli N, Garcia RB, et al. Evaluation of the physical and chemical properties of two commercial and three experimental root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(2):250-6.
[https://doi.org/10.1016/j.tripleo.2010.04.021]

-
Jun SK, Yoon JY, Mahapatra C, Park JH, Kim HW, Kim HR, et al. Ceria-incorporated MTA for accelerating odontoblastic differentiation via ROS downregulation. Dent Mater. 2019;35(9):1291-9.
[https://doi.org/10.1016/j.dental.2019.05.024]

-
Vitti RP, Prati C, Sinhoreti MA, Zanchi CH, Souza ESMG, Ogliari FA, et al. Chemical-physical properties of experimental root canal sealers based on butyl ethylene glycol disalicylate and MTA. Dent Mater. 2013;29(12):1287-94.
[https://doi.org/10.1016/j.dental.2013.10.002]

-
Almeida LHS, Moraes RR, Morgental RD, Cava SS, Rosa WLO, Rodrigues P, et al. Synthesis of silver-containing calcium aluminate particles and their effects on a MTA-based endodontic sealer. Dent Mater. 2018;34(8):e214-e23.
[https://doi.org/10.1016/j.dental.2018.05.011]

-
Vitti RP, Prati C, Silva EJ, Sinhoreti MA, Zanchi CH, de Souza e Silva MG, et al. Physical properties of MTA Fillapex sealer. J Endod. 2013;39(7):915-8.
[https://doi.org/10.1016/j.joen.2013.04.015]

-
Camilleri J. Sealers and warm gutta-percha obturation techniques. J Endod. 2015;41(1):72-8.
[https://doi.org/10.1016/j.joen.2014.06.007]

-
Prullage RK, Urban K, Schafer E, Dammaschke T. Material properties of a tricalcium silicate-containing, a mineral trioxide aggregate-containing, and an epoxy resin-based root canal sealer. J Endod. 2016;42(12):1784-8.
[https://doi.org/10.1016/j.joen.2016.09.018]

-
Formosa LM, Mallia B, Camilleri J. Mineral trioxide aggregate with anti-washout gel - properties and microstructure. Dent Mater. 2013;29(3):294-306.
[https://doi.org/10.1016/j.dental.2012.11.009]

-
Siboni F, Taddei P, Prati C, Gandolfi MG. Properties of NeoMTA Plus and MTA Plus cements for endodontics. Int Endod J. 2017;50 Suppl 2:e83-e94.
[https://doi.org/10.1111/iej.12787]

-
Taylor HFW. Cement chemistry. 2nd ed. London: Academic Press; 1997.
[https://doi.org/10.1680/cc.25929]

-
Camilleri J, Sorrentino F, Damidot D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent Mater. 2013;29(5):580-93.
[https://doi.org/10.1016/j.dental.2013.03.007]

-
Huan Z, Chang J. Study on physicochemical properties and in vitro bioactivity of tricalcium silicate-calcium carbonate composite bone cement. J Mater Sci Mater Med. 2008;19(8):2913-8.
[https://doi.org/10.1007/s10856-008-3423-4]

-
Paiva H, Silva AS, Velosa A, Cachim P, Ferreira VM. Microstructure and hardened state properties on pozzolan-containing concrete. Constr Build Mater. 2017;140:374-84.
[https://doi.org/10.1016/j.conbuildmat.2017.02.120]

-
Lv Y, Niu Y, Kan H, Wang D, et al. Experimental comparative study on ash fusion characteristics of Ningdong coal under oxidizing and reducing atmosphere by means of SiO2-Al2O3-(CaO+ MgO+ Na2O+ K2O) pseudo-ternary diagrams. Fuel 2019;258:116137.
[https://doi.org/10.1016/j.fuel.2019.116137]

-
Costa BC, Guerreiro-Tanomaru JM, Bosso-Martelo R, Rodrigues EM, Bonetti-Filho I, Tanomaru-Filho M. Ytterbium oxide as radiopacifier of calcium silicate-based cements. Physicochemical and biological properties. Braz Dent J. 2018;29(5):452-8.
[https://doi.org/10.1590/0103-6440201802033]

-
Ginebra MP, Driessens FC, Planell JA. Effect of the particle size on the micro and nanostructural features of a calcium phosphate cement: a kinetic analysis. Biomaterials. 2004;25(17):3453-62.
[https://doi.org/10.1016/j.biomaterials.2003.10.049]

-
Ha WN, Kahler B, Walsh LJ. The influence of particle size and curing conditions on testing mineral trioxide aggregate cement. Acta Biomater Odontol Scand. 2016;2(1):130-7.
[https://doi.org/10.1080/23337931.2016.1239181]

-
Ha WN, Bentz DP, Kahler B, Walsh LJ. D90: the strongest contributor to setting time in mineral trioxide aggregate and Portland cement. J Endod. 2015;41(7):1146-50.
[https://doi.org/10.1016/j.joen.2015.02.033]