
The Influence of Diabetes Mellitus on Expression of Stromelysins and Membrane type Matrix Metalloproteinases in Human Chronic Periodontitis
The purposes of this study were to observe influence of diabetes mellitus(DM) on the expression of MMP-3 of stromelysin type and MMP-14 of membrane type matrix metalloproteinase in the gingival tissues of patients with type 2 DM and healthy adults with chronic periodontitis. Gingival samples were devided into three groups. Group 1 is clinically healthy gingiva without bleeding, bone resorption or periodontal pockets. Group 2 is inflammed gingiva from patients with chronic periodontitis. Group 3 is inflammed gingiva from patients with chronic periodontitis associated with type 2 DM. Tissue samples were analyzed by Western blotting. The quantification of MMP-3 and MMP-14 were performed using a densitometer. MMP-3 and MMP-14 expressions were similar in group 1 and 2. While expression of MMP-14 increases in proportion to MMP-3 expression, expression of both type of MMP showed increasing tendency in chronic periodontitis associated to type 2 DM and mean amount of it was more increased in group 3 comparing to other group with statistically significant difference.
In conclusion, MMP-3 and MMP-14 expression levels were similar in healthy and inflamed gingival tissue from systemically healthy patients. And tissue with chronic periodontitis associated to type 2 DM showed significantly increased MMP-3 and MMP-14 levels compared to healthy and non-diabetic inflamed gingiva. Also, MMP-3 may be related to MMP-14 activity in chronic periodontitis associated with type 2 DM. Therefore, the expression levels of MMP-3 and MMP-14 will be inflammatory markers of periodontal inflammed tissue with type 2 DM.
Keywords:
chronic periodontitis, MMP-3, MMP-14, Type 2 Diabetes MellitusINTRODUCTION
Periodontitis are infectious diseases characterized by periodontal attachment loss and bone destruction (Genco et al., 1998; Socransky et al., 1992). The components of the periodontal tissue extracellular matrix (ECM), especially collagens, appear to be the main targets of degradation in periodontitis. Among host proteases degrading the extra-cellular matrix, matrix metalloproteinases (MMPs) seem to be highly related to tissue destruction and remodelling events in periodontal disease (Reynolds J, 1995; Van der Zee et al., 1997).
MMPs are normally under tight regulation, not only at the level of gene expression but also extracellularly after secretion. Disruption of this regulation leads to the pathologic breakdown of connective tissues. High levels of MMPs on the periodontal tissues provoke an imbalance between the production and degradation of collagen, causing tooth attachment loss (Kumar et al., 2006) .
The MMPs are divided into 4 major subclasses based on their substrate specificity and sequence homology (Werb Z, 1997) :(a) collagenases type; (b) gelatinases type, also called type IV collagenases; (c) stromelysins such as MMP-3; (d) the recently discovered MT-MMPs (membrane type matrix metalloproteinase) such as MMP-14.
One prominent member of this family, MMP-3 of stromelysin type has been shown to be the product of a variety of cells, including monocytes, endothelial cells, chondrocytes and synoviocytes, as well as gingival fibroblasts (Domeij et al., 2002). MMP-3 is effective in the degradation of numerous ECM substrates including gelatins, proteoglycans, laminin, fibronectin and types IV and IX collagen (Nakaya et al., 1997). In addition to its ability to degrade various connective tissue components, MMP-3 is also known to participate in the proteolytic activation cascades of latent pro-MMP-1, -8 and -9 (Ogata et al., 1992; Sorsa et al., 1990; Suzuki et al., 1990). MMP-3 mediated collagenolysis may be a major pathway in the destruction and remodelling of connective tissue in periodontitis.
In 1994, Sato et al. (1994) cloned the first membrane-type MMP (MT1-MMP, MMP-14) and demonstrated it to be an activator of pro-MMP-2. MMP-14 was later shown to degrade various ECM components, including type I, II and III collagen, fibronectin, laminins and proteoglycans (Koshikawa et al., 2000; Sato et al., 2005; Seiki et al., 2002). MMP-14 is not released but rather acts in the cell membrane and activates pro‐-MMP-8, and -13. Achong et al. (2003) demonstrated that during the remodeling and healing phases of a periodontal lesion, MMP-14 with activated MMP-2 at the cell membrane may allow cell migration and the restructuring of the ECM.
Type I and type II diabetes mellitus (DM) have been associated with unusual periodontal diseases modified by the systemic condition. It may also be interesting to note that severe periodontitis in diabetics is associated with a greater risk of renal, cardiovascular and infectious complications (Thorstensson et al., 1996). Research has been done on the susceptibility to infections and compromised wound healing among patients with DM (Rayfield et al., 1982; Terranova A, 1991). DM does not cause periodontitis itself, but it is particularly associated with a higher prevalence of periodontal disease, especially concerning the degree of metabolic control (Grossi & Genco, 1998; Kinane & Chestnutt, 1997). Previous studies showed that impaired polymorphonuclear leukocyte(PMN) functions were linked to periodontitis and diabetes, as the chemotactic response of PMN cells was mostly compromised in diabetic subjects with periodontitis compared to diabetic patients with mild periodontitis or healthy subjects with either mild or severe periodontitis (Bissada et al., 1982).
In inflammatory response, the roles and interactions of stromelysins and membrane type are not clear. The relative contribution of MMP-3 of stromelysin type and MMP-14 of membrane type in the pathogenesis of periodontitis is still not entirely established. Moreover non of the in vivo studies simultaneously analysed each MMP-3 of stromelysin type and MMP-14 of membrane type and it's interrelationship for the diabetic and nondiabetic patients with chronic periodontitis. The purposes of this study were to observe influence of diabetes mellitus on the expression of MMP-3 of stromelysin type and MMP-14 of membrane type in the gingival tissues of patients with type 2 diabetes mellitus and healthy adults with chronic periodontitis.
MATERIALS AND METHODS
1. Study population and tissue sampling
Study population consisted of 8 patients with type 2 diabetes and chronic periodontitis, 8 patients with chronic periodontitis, and 8 healthy individuals. Marginal gingival tissue samples were obtained by internal bevel incision at the time of periodontal surgery (including surgical crown lengthening) or tooth extraction and informed consent was obtained from all of the participants before the surgery.
Clinical criteria of gingiva (Sulcus bleeding index value (Muhlemann & Son, 1971), probing depths) and radiographic evidences of bone resorption, each gingival sample was divided into the three groups. Group 1 (normal, n=8) is clinically healthy gingiva without bleeding and no evidence of bone resorption or periodontal pockets, obtained from systemically healthy 8 patients. Group 2 (chronic periodontitis, n=8) is inflamed gingiva from patients with chronic periodontitis. The diagnosis of chronic periodontitis was established on the basis of clinical and radiographic criteria (bone resorption) according to the classification system for periodontal disease and condition (Amitage GC, 1999). All patients of group 2 were systemically healthy and had more than one periodontal pockets ≥5 mm and at least one pocket with ≥4mm loss of attachment. All gingival samples were obtained from the teeth with probing depth ≥5 mm, swelling of the marginal gingiva, and bleeding corresponding to gingival sulcus bleeding indexes 3 according to Mühlman and Son (1971). Group 3 (chronic periodontitis & type 2 DM, n=8) is inflamed gingiva from patients with chronic periodontitis associated with type 2 diabetes. Patients in group 2 & 3 have similar periodontal condition, but patients in group 2 were systemically healthy and patients in group 3 had type 2 diabetes with treatment. Patients in group 3 were diagnosed type 2 diabetes mellitus since 6 months and showed above 200 mg/dl blood glucose level in postprandial 2 hours. Gingival sample were obtained by similar way described above.
Following surgery, excised tissue specimens were immediately placed on liquid nitrogen and subsequently frozen (-70℃).
2. Protein isolation and Western blotting
For Western blotting, as previously described technique by Park et al. (2006) frozen tissues were homogenized in RIPA lysis buffer (10 mM EDTA, 0.15M NaCl) with 1:30 diluted protease inhibitor cocktail (Roche, Germany) according to Cho's method (Cho et al., 2000). The lysates were sonicated 3 times for 10 seconds and centrifuge at 12,000g for 15 minutes. Protein concentrations of supernant were routinely determined by a Braford protein asssay (Quick Start, BIO-RAD, USA) using BSA as standard.
Lysates were boiled in SDS samples buffer (1M Tris-Cl (pH6.8), 40% glycerol, 8% SDS, 2% mercapto-ethanol, 0.002% Bromophenole blue). Prepared samples were separated by 15 % sodium dodecyl sulfate (SDS)- polyacrylamide gels and transferred to a polyvinylidene difluride membrane.
The membranes were subsequently blocked in Trisbuffered saline (TBS) containing 5% powdered milk and 1% BSA for 1 hour, and then incubated with polyclonal anti-MMP-3, and anti-MMP-14 (Prepared in rabbit, diluted 1: 1,000 and 1: 2,000 in TBS, respectively, Sigma-Aldrich, Inc. USA) antibody for 1.5 hours at room temperature.
The membranes were washed (five times for 5 minutes with Tween 20) and incubated with a horseradish peroxidase(HRP)-conjugated goat anti-rabbit secondary antibody for anti-MMP-3 and anti-MMP-14 (diluted 1: 2,000 in TBS) antibody for 1 hour at room temperature. After additional washing (five times for 5 minutes with Tween 20), the Western blot procedure was completed with an ECL Plus development kit (Amsterdam, Beckinghamshire, U.K.)
The quantification analysis of MMP-3 and MMP-14 expression was performed using a densitometer (Scion Image β 4.02, Scion Corporation, USA). After normalization to β-actin (Abcam® U.K.) in each sample, level of MMP-3 and MMP-14 were expressed as a ratio of MMP-3 or MMP-14/β-actin and the differences of density between 3 groups were determined.
3. Statistical analysis of the Western blot results
All data were presented as means ± standard deviation and results were statistically analyzed. The MMP-3 and MMP-14 levels among each 3 groups were compared using one way ANOVA followed by Tukey test. P value < 0.05 was considered to statistically significant.
RESULTS
Both chronic periodontitis group & chronic periodontitis with type 2 DM group showed the expression of MMP-3 and MMP-14 in all samples. To compare MMP-3 expression levels in human gingiva with chronic periodontitis with or without associated to Type 2 diabetes mellitus, MMP-3 specific antibodies were used to detect the cytokine in the tissues (Fig. 1A, B). Representative Western blot data were presented in Fig. 1A which detected about 59kDa molecular weight of MMP-3 in all three groups. The expression levels of β-actin were also measured by anti-β-actin specific western blot analysis. In order to quantify the level of MMP-3 expression in the groups, the expression levels of MMP-3 in each sample were measured by densitometer. Then MMP-3 expression levels were normalized by β-actin (ratio of MMP-3/β-actin). The levels of gingival normalized MMP-3 expression were summarized as a graph in Fig. 1B.
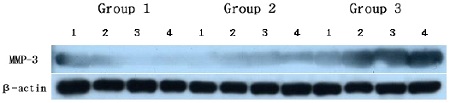
MMP-3 Western analysis showing 4 representative samples in each group. MMP-3 corresponding to molecular weight 59kDa was shown to be expressed in all samples including healthy gingiva. The expression levels of MMP-3 were increased in order of group 1, group 2, group 3. In order to quantify detected MMP-3, β-actin levels were also measured. Group 1 : healthy gingiva from systemically healthy person Group 2 : inflammed gingiva from patient with chronic periodontitisGroup 3 : inflammed gingiva from patient with chronic periodontitis and type DM
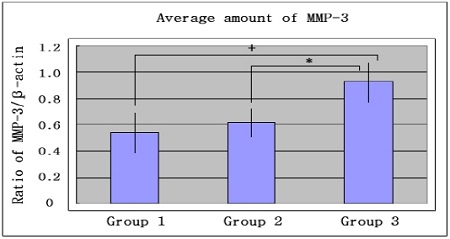
Graphics showing the average amounts (Ratio of MMP-3/β-actin) and standard deviation of MMP-3 in group 1, 2 and 3. In the inflammed gingiva with diabetes (group 3), MMP-3 seemed to be increased compared to group 1 and group 2. Group 1 : healthy gingiva from systemically healthy personGroup 2 : inflammed gingiva from patient with chronic periodontitisGroup 3 : inflammed gingiva from patient with chronic periodontitis and type 2 DM+ significant difference between group 1 and group 3 (P<0.05)* significant difference between group 2 and group 3 (P<0.05)
The mean amount of MMP-3 expression (ratio of MMP-3/β-actin) were 0.545±0.177 in group 1, 0.617 ±0.117 in group 2 and 0.934±0.166 in group 3. There was no significant difference between group 1 and group 2, but the differences between group 1 and group 3 and between group 2 and group 3 were statistically significant (p<0.05).
The comparison of MMP-14 expression levels were also studied by Western blot analysis using MMP-14 specific antibody which detected about 63kDa molecular weight of MMP-14 in all three groups (Fig. 2A). The levels of MMP-14 expression were also quantified with β-actin normalization (Fig. 2B). The mean amounts of MMP-14 expression (ratio of MMP-14 /β-actin) were 0.582±0.178 in group 1, 0.637±0.123 in group 2 and 1.060±0.217 in group 3. There was no significant difference between group 1 and group 2, but the differences between group 1 and group 3 and between group 2 and group 3 were statistically significant (p<0.05).

MMP-14 Western analysis showing 4 representative samples in each group. MMP-14 corresponding to molecular weight 63kDa was shown to be expressed in all samples including healthy gingiva and the expression levels of MMP-14 were increased in order of group 1, group 2, group 3. In order to quantify detected MMP-14, β-actin levels were also measured. Group 1 : healthy gingiva from systemically healthy personGroup 2 : inflammed gingiva from patient with chronic periodontitisGroup 3 : inflammed gingiva from patient with chronic periodontitis and type DM
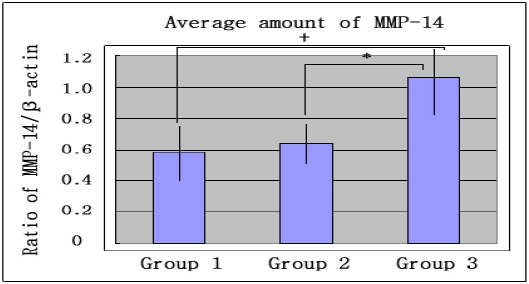
Graphics showing the average amounts (Ratio of MMP-14/β-actin) and standard deviation of MMP-14 in group 1, 2 and 3. In the inflammed gingiva with diabetes (group 3), MMP-14 seemed to be increased compared to group 1 and group 2. Group 1 : healthy gingiva from systemically healthy personGroup 2 : inflammed gingiva from patient with chronic periodontitisGroup 3 : inflammed gingiva from patient with chronic periodontitis and type 2 DM+ significant difference between group 1 and group 3 (P<0.05)* significant difference between group 2 and group 3 (P<0.05)
In the interrelationship of MMP-3 and MMP-14 expressions, expressions of MMP-3 and MMP-14 showed increasing tendency in chronic periodontitis associated to type 2 DM and it seems that the MMP-14 expressions were increasing in proportion to MMP-3 expressions.
DISCUSSION
Numerous studies have demonstrated the association of diabetes with periodontal diseases in human subjects. Also, periodontal disease progresses more rapidly and leads to more tooth loss in pooly controlled patients (Portik-Dobos et al., 2002; Uemura et al., 2001). Various pathogenetic factors have been suggested to explain the increased prevalence and severity of periodontitis in diabetes (Emrich et al., 1991; Taylor et al., 1998).
Collagen, as the central component in wound healing, represents the quantitatively most frequent protein of the body and the most important scleroprotein of the gingiva (Chavrier et al., 1984). MMPs are involved in a number of physiological events, including tissue remodeling, and pathological processes such as periodontal disease. MMPs are the major players in collagen breakdown during periodontal tissue destruction (Van der Zee et al., 1996).
The purpose of this study was to quantify and compare the expression of MMP-3 of stromelysin type and MMP-14 of membrane type in the gingival tissue of the patients with chronic periodontitis associated to type 2 DM, in order to understand the contribution of these proteins to periodontal destruction in type 2 diabetic patients, especially MMP-mediated host response in type 2 diabetic patients.
In this study, MMP-3 and MMP-14 expressions were similar in normal tissue and inflamed tissue from systemically health patients. It is likely that this finding was caused by various degrees of inflammation in obtained tissue samples.
Until now, few studies reports on the expression tendency of MMP-3 of stromelysin type in periodontitis patient associated to type 2 DM. In this study MMP-3 corresponding to molecular weight 59 kDa was expressed in most samples. The quantitative analysis of MMP-3 level showed that MMP-3 expression was rather increased in inflamed gingiva associated to type 2 DM compared to healthy gingiva and inflamed gingiva of systemically healthy patient. And the difference was statistically significant. This result indicates MMP-3 reveals different response tendency in disease progression in type 2 DM patient and plays role in part in increased inflammatory response of patient with this systemic disease.
Diabetes is characterized by hyperglycemia. Prolonged exposure to hyperglycemia is the primary factor responsible for the development of diabetic complications (Brownlee M, 1992; Brownlee M, 1994; The Diabetes Control and Complications Trial Research Group, 1993). The common biochemical basis is hyperglycemia-mediated formation of non-enzymatic advanced glycation end products (AGEs) (Brownlee & Cerami, 1981). Glucosemediated AGE accumulation would affect migration and phagocytic activity of mononuclear and polymorphonuclear phagocytic cells, resulting in the establishment of a more pathogenic subgingival flora. This, in turn, triggers both an "infection-mediated" pathway of cytokine upregulation, especially with secretion of TNF-α and IL-1 (Sara et al., 1998). In gingival fibroblasts, Wassenaar et al. & Tewari et al. demonstrated that MMP-3 expression is stimulated by TNF-α and IL-1 (Tewari etal., 1994; Wassenaar et al., 1999).
Adult periodontitis crevicular fluid contains elevated levels of neutrophil-derived cathepsin G (Tervahartiala et al., 1996) and elastase (Ingman et al., 1994). They are able to activate pro-MMP-3 (Jenne DE, 1994; . Okada & Nakanishi, 1989). It is therefore proposed that they activate gingival fibroblast-derived pro-MMP-3 in GCF. Pro-MMP-8 and pro-MMP-9 are also released from activated, degranulating neutrophils (Weiss SJ, 1989). Active MMP-3 then cleaves off the 10-kDa activation peptide blocking the active site zinc in pro-MMP-8 and pro-MMP-9. This would guarantee co-ordinated activation of pro-collagenase-8(MMP-8) and pro-gelatinase B (MMP-9), which exert consecutive actions upon degradation of type I collagen and gelatin, respectively (Beklen et al., 2006).
The amounts of MMP-14 expression was higher in chronic periodontitis with type 2 DM compared to chronic periodontitis group of systemic healthy person and healthy gingiva from systemically healthy person. And there was statistically significant difference.
Fibroblasts may be a major contributor to the elevated levels of MMPs seen on chronic wounds. Subepithelial fibroblasts in inflamed gingiva are the main cells that produced MMP-14 (Trengove et al., 1999). As previously demonstrated, hyperglycemia in diabetic condition is able to induce various cytokine upregulation. These cytokine may stimulate gingival fibroblasts. But it was impossible to figure out the exact signaling mechanism, so further to examine inflammatory response of MMP-14 of membrane type and other mediator in gingival tissue needed.
Ohuchi et al. (1997) demonstrated that MMP-14 is an extracellular matrix degrading enzyme sharing the substrate specificity with interstitial collagenases and may have a dual role in pericellular ECM degradation through direct cleavage of the substrates and activation of pro-MMP-2 under the pathological conditions. Moreover, MMP-14, expressed either in the basal cells of sulcular epithelium or in adjacent gingival connective tissue, may serve as procollagenase (MMP-13) activator (Kuauper et al., 1996; Von Bredow et al., 1998).
In interrelationship between MMP-3 and MMP-14, it is known that pro-MMP-3 and MMP-3 stimulates plasminogen activation by tissue-type and urokinase-type plasminogen activator, respectively (Arza et al., 2000; Ugwu et al., 1998). Plasminogen is converted into the active enzyme plasmin by plasminogen activator following cleavage of the Arg561-Val562 peptide bond (Robbins et al., 1967). Accodingly, plasmin activates the precursor of MMP-14 (Okumura et al., 1997) and activates pro-MMP-2 with a MMP-14-dependent mechanism (Monea et al., 2002). As previously demonstrated, hyperglycemia increase glucose-mediated AGE accumulation in diabetic condition and AGE may induce MMP-3 expression in gingival fibroblasts by a cytokine upregulation. Therefore, it is assumed that MMP-3 may stimulates MMP-14 activity by plasmin in diabetes. In this study, expressions of MMP-3 and MMP-14 showed increasing tendency in chronic periodontitis associated to type 2 DM and it seems that the MMP-14 expressions were increasing in proportion to MMP-3 expressions. It is suggested that MMP-3 may be related to MMP-14 activity in chronic periodontitis associated with type 2 DM. But, it is also considered that other cytokines or other tissue degradation enzymes are involved complexly in MMP-14 expression.
In conclusion, this study demonstrated that MMP-3 of stromelysin type and MMP-14 of membrane type expression levels in human gingival tissue were similar healthy and inflamed tissue from systemically health patients. And tissue with chronic periodontitis associated to type 2 DM showed significantly increased MMP-3 and MMP-14 levels compared to healthy gingiva and non-diabetic inflamed gingiva. Also, interrelationship between MMP-3 and MMP-14, MMP-3 may be related to MMP-14 activity in chronic periodontitis associated with type 2 DM. It is suggested that MMP-3 and MMP-14 may be partly involved in the progression of periodontal inflammation associated with type 2 DM, as related to a metabolism of other factors, such as AGE, plasmin and other MMPs.
The dental implants can occur the periimplantitis in the same manner as natural teeth and bone graft material could be related to the inflammatory response. Thus, it is considered to be necessary to select the material which can show the least inflammatory response possible when it confronts the expression of MMP-3 and MMP-14.
Finally, it seemed that more studies are needed to investigate the effect and interrelationship between MMPs and other cytokines that affect the progression of periodontal disease at a higher level and these studies seems to be able to contribute to the development of disease diagnosis methods and treatment modality.
CONCLUSION
The purposes of this study were to observe influence of DM on the expression of MMP-3 of stromelysin type and MMP-14 of membrane type in the gingival tissues of patients with type 2 diabetes mellitus (DM) and healthy adults with chronic periodontitis.
Gingival tissue samples were obtained during periodontal surgery or tooth extraction. According to the patient's systemic condition & clinical criteria of gingiva, each gingival sample was devided into three groups. Group 1 is clinically healthy gingiva without bleeding and no evidence of bone resorption or periodontal pockets, obtained from systemically healthy 8 patients. Group 2 is inflammed gingiva from patients with chronic periodontitis. Group 3 is inflammed gingiva from patients with chronic periodontitis associated with type 2 DM. Tissue samples were prepared and analyzed by Western blotting. The quantification of MMP-3 and MMP-14 were performed using a densitometer and statistically analyzed by one-way ANOVA followed by Tukey test.
In the analysis of expression levels, MMP-3 and MMP-14 expressions were similar in group 1 and 2. And mean amount of MMP-3 and MMP-14 was more increased in group 3 than group 1, 2. The difference between group 3 and group 1, 2 was statistically significant. Also, in the interrelationship of MMP-3 and MMP-14 expressions, expressions of MMP-3 and MMP-14 showed increasing tendency in chronic periodontitis associated to type 2 DM and it seems that the MMP-14 expressions were increasing in proportion to MMP-3 expressions.
In conclusion, this study demonstrated that MMP-3 of stromelysin type and MMP-14 of membrane type expression levels in human gingival tissue were similar healthy and inflamed tissue from systemically health patients. And tissue with chronic periodontitis associated to type 2 DM showed significantly increased MMP-3 and MMP-14 levels compared to healthy gingiva and non-diabetic inflamed gingiva. Also, interrelationship between MMP-3 and MMP-14, MMP-3 may be related to MMP-14 activity in chronic periodontitis associated with type 2 DM. It is suggested that MMP-3 and MMP-14 may be partly involved in the progression of periodontal inflammation associated with type 2 DM, as related to a metabolism of other factors, such as AGE, plasmin and other MMPs. Therefore, the expression levels of MMP-3 and MMP-14 will be inflammatory markers of periodontal inflammed tissue with type 2 DM.
References
-
Achong, R., Nishimura, I., Ramachandran, H., Howell, TH., Fiorellini, JP., Karimbux, NY., (2003), Membrane Type(MT)1-matrix metalloproteinase(MMP) and MMP-2 expression in ligature-induced periodontitis in the rat, J Periodont, 74, p494-500 .
[https://doi.org/10.1902/jop.2003.74.4.494]

- Amitage, GC., (1999), Development of a classification system for periodontal diseases and conditions, Ann Periodontol, 4, p1-6 .
-
Arza, B., Hoylaerts, MF., Felez, J., Collen, D., Lijnen, HR., (2000), Prostromelysin-1 (proMMP-3) stimulates plasminogen activation by tissue-type plasminogen activator, Eur J Biochem, 267, p6378-6384 .
[https://doi.org/10.1046/j.1432-1327.2000.01732.x]

-
Beklen, A., Tuter, G., Sorsa, T., Hanemaaijer, R., Virtanen, I., Tervahartiala, T., Konttinen, YT., (2006), Gingival tissue and crevicular fluid co-operation in adult periodontitis, J Dental Res, 85(1), p59-63 .
[https://doi.org/10.1177/154405910608500110]

-
Bissada, NF., Manouchehr-Pour, M., Haddow, M., Spagnuolo, PJ., (1982), Neutrophil functional activity in juvenile and adult onset diabetic patients with mild and severe periodontitis, J Periodontal Res, 17, p500-502 .
[https://doi.org/10.1111/j.1600-0765.1982.tb02038.x]

-
Brownlee, M., (1992), Glycation products and the pathogenesis of diabetic complications, Diabetes Care, 15, p1835-1843 .
[https://doi.org/10.2337/diacare.15.12.1835]

-
Brownlee, M., (1994), Glycation and diabetic complications, Diabetes, 43, p836-841 .
[https://doi.org/10.1146/annurev.bi.50.070181.002125]

-
Brownlee, M., Cerami, A., (1981), The biochemistry of the complications of diabetes mellitus, Ann Rev Biochem, 50, p385-432 .
[https://doi.org/10.1111/j.1600-0765.1984.tb00813.x]

-
Chavrier, C., Couble, ML., Magloire, H., Grimaud, JA., (1984), Connective tissue organization of healthy human gingiva. Ultrastructural localization of collagen types I-III-IV, J Periodontal Res, 19, p221-229 .
[https://doi.org/10.1038/sj.gt.3301170]

-
Cho, JY., Xing, S., Liu, X., Buckwalter, TLF., Hwa, L., Sferra, TJ., Chiu, IM., Jhiang, SM., (2000), Expression and activity of human Na+/I- symporter in human glioma cells by adenovirus-mediateed gene delivery, Gene Therapy, 7, p740-749 .
[https://doi.org/10.1034/j.1600-0722.2002.21247.x]

-
Domeij, H., Yucel-Lindberg, T., Modeer, T., (2002), Signal pathways involved in the production of MMP-1 and MMP-3 in human gingival fibroblasts, European Journal of Oral Science, 110, p302-306 .
[https://doi.org/10.1902/jop.1991.62.2.123]

- Emrich, LJ., Shlosman, M., Genco, RJ., (1991), Periodontal disease in non-insulin dependent diabetes mellitus, J Periodont, 62, p123-129 .
-
Genco, RJ., Zambon, JJ., Christersson, LA., (1998), The origin of periodontal infection, Advances in Dental Research, 2, p245-259.
[https://doi.org/10.1902/annals.1998.3.1.51]

-
Grossi, SG., Genco, RJ., (1998), Periodontal disease and diabetes mellitus: A two-way relationship, Ann periodontol, 3, p51-61 .
[https://doi.org/10.1111/j.1600-0722.1994.tb01481.x]

-
Ingman, T., Sorsa, T., Michaelis, J., Konttinen, YT., (1994), Immunohistochemical study of neutrophil- and fibroblast-type collagenases and stromelysin-1 in adult periodontitis, J Dental Research, 102, p342-349 .
[https://doi.org/10.1164/ajrccm/150.6_Pt_2.S147]

- Jenne, DE., (1994), Structure of the azurocidin, proteinase 3, and neutrophil elastase genes. Implications for inflammation and vasculitis, Am J Respir Crit Care Med, 150(6 Pt 2), ps147-s154 .
-
Kinane, DF., Chestnutt, IG., (1997), Relationship of diabetes to periodontitis, Curr Opin Periodontol, 4, p29-34 .
[https://doi.org/10.1083/jcb.148.3.615]

- Koshikawa, N., Giannelli, G., Cirulli, V., Miyazaki, K., Quaranta, V., (2000), Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5, J. Cell Biol, 148, p615-624 .
-
Kuauper, V., Will, H., Lopez-Otin, C., Smith, B., Atkinson, SJ., Stanton, H., (1996), Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP(MMP-14) and gelatinase A(MMP-2) are able to generate active enzyme, J Biol Chem, 271, p17124-17131 .
[https://doi.org/10.1902/jop.2006.050293]

- Kumar, MS., Vamsi, G., Sripriya, R., Sehgal, PK., (2006), Expression of matrix metalloproteinases(MMP-8 and -9) in chronic periodontitis patients with and without diabetes mellitus, J Periodont, 77(11), p1803-1808 .
- Monea, S., Lehti, K., Keski-oja, J., Mignatti, P., (2002), Plasmin activates pro-matrix metalloproteinase-2 with a membrane-type 1 matrix metalloproteinasedependent mechanism, J of cellular physiology, 192, p160-170 .
- Muhlemann, HR., Son, S., (1971), Gingival sulcus bleeding a leading symptom in initial gingivitis, Helv Odontol Acta, 15, p107.
- N. Engl, (1993), The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complication in insulindependent diabetes mellitus, J Med, 329, p977-986 .
- Nakaya, H., Oates, TW., Hoang, AM., Kamoi, K., Cochran, DL., (1997), Effects of IL-1 beta on matrix metalloproteinase 3 levels in human periodontal ligament cells, J Periodont, 68, p517-523 .
- Ogata, Y., Enghild, JJ., Nagase, H., (1992), Matrix metalloproteinase 3 (stromelysin) activates the precursors for human matrix metalloproteinase 9, J Biol Chem, 267, p3581-3584 .
-
Ohuchi, E., Imai, K., Fujii, Y., Sato, H., Seiki, M., Okada, Y., (1997), Membrane type-1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules, J Biol Chem, 272, p2446-2451 .
[https://doi.org/10.1016/0014-5793(89)80657-X]

- Okada, Y., Nakanishi, I., (1989), Activation of matrix metalloproteinase 3 (stromelysin) and matrix metalloproteinase 2 (gelatinase) by human neutrophil elastase and cathepsin G, FEBS Lett, 249, p353-356 .
-
Okumura, Y., Sato, H., Seiki, M., Kido, H., (1997), Proteolytic activation of the precursor of membrane type 1 matrix metalloproteinase by human plasmin: A possible cell surface activator, FEBS Letters, 402, p181-184 .
[https://doi.org/10.5051/jkape.2006.36.2.397]

- Park, HK., Lee, JM., (2006), Interrelationship of matrix metalloproteinase-13 and elastase expression in human gingiva with chronic periodontitis associated to type 2 diabetes mellitus, The Journal of Korean Academy of Periodontology, 36, p397-408 .
-
Portik-Dobos, V., Anstadt, MP., Hutchinson, J., Bannan, M., Ergul, A., (2002), Evidence for a matrix metalloproteinase induction/activation system in arterial vasculature and decreased synthesis and activity in diabetes, Diabetes, 51, p3063-3068 .
[https://doi.org/10.1016/0002-9343(82)90511-3]

-
Rayfield, EJ., Ault, MJ., Keusch, GT., Brothers, MJ., Nechemias, C., (1982), Smith, H.., Infection and diabetes: The case for glucose control, Am J Med, 72, p439-450 .
[https://doi.org/10.1111/j.1601-0825.1996.tb00206.x]

- Reynolds, JJ., (1995), Collagenases and tissue inhibitors of metalloproteinases : a functional balance in tissue degradation, Oral Diseases, 2, p70-76 .
- Robbins, KC., Summaria, L., Hsieh, B., Shah, RJ., (1967), The peptide chains of human plasmin. Mechanism of activation of human plasminogen to plasmin, J Biol Chem, 242, p2333-2342 .
- Sara, G Grossi, Robert, J Genco, (1998), Periodontal disease and diabetes mellitus: A two-way relationship, Annals of Periodontology, 3, p51-61 .
-
Sato, H., Takino, T., Miyamori, H., (2005), Roles of membrane-type matrix metalloproteinase-1 in tumor invation and metastasis, Cancer Sci, 96, p212-217 .
[https://doi.org/10.1038/370061a0]

-
Sato, H., Takino, T., Okada, Y., Cao, J., Shinagawa, A., Yamamoto, E., Seiki, M., (1994), A matrix metalloproteinase expressed on the surface of invasive tumour cells, Nature, 370, p61-65 .
[https://doi.org/10.1016/S0955-0674(02)00363-0]

-
Seiki, M., (2002), The cell surface: the stage for matrix metalloproteinase regulation of migration, Curr Opin Cell Biol, 14, p624-632 .
[https://doi.org/10.1902/jop.1992.63.4s.322]

-
Socransky, SS., Haffajee, AD., (1992), The bacterial etiology of destructive periodontal disease: current concepts, J Periodont, 63, p322-331 .
[https://doi.org/10.1016/0003-9969(90)90156-5]

-
Sorsa, T., Suomalainen, K., Uitto, VJ., (1990), The role of gingival crevicular fluid and salivary interstitial collagenases in human periodontal diseases, Archives of Oral Biology, 35, p193-196 .
[https://doi.org/10.1021/bi00496a016]

- Suzuki, K., Enghild, JJ., Mordomi, T., Salvesen, G., Nagase, H., (1990), Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin), Biochemistry, 29, p10261-10270 .
-
Taylor, GW., Burt, BA., Becker, MP., Genco, RJ., Shlossman, M., Knowler, WC., Pettitt, DJ., (1998), Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years, J Periodont, 69, p76-83 .
[https://doi.org/10.1097/00006527-199101110-00006]

-
Terranova, A., (1991), The effects of diabetes mellitus on wound healing, Plast Surg Nurs, 11, p20-25 .
[https://doi.org/10.1111/j.1600-051X.1996.tb00537.x]

-
Tervahartiala, T., Konttinen, YT., Ingman, T., Hayrinen-IMMonen, R., Ding, Y., Sorsa, T., (1996), Cathepsin G in gingival tissue and crevicular fluid in adult periodontitis, J Clin Periodontol, 23, p68-75 .
[https://doi.org/10.1016/0003-9969(94)90091-4]

- Tewari, DS., Qian, Y., Tewari, M., Pieringer, J., Thornton, RD., Taub, R., Mochan, EO., (1994), Mechanistic features associated with induction of metalloproteinases in human gingival fibroblasts by interleukin-1, Arch Oral Biol, 39, p657-664 .
- Thorstensson, H., Kuylenstiern, J., Hugoson, A., (1996), Medical status and complications in relation to periodontal disease experience in insulin-dependent diabetics, J Clin Periodontol, 23, p194-202 .
-
Trengove, NJ., Stacey, MC., MacAuley, S., (1999), Analysis of the acute and chronic wound environments: the role of proteases and the inhibitors, Wound Repair Regeneration, 7, p442-52 .
[https://doi.org/10.1021/bi9728708]

- Uemura, S., Matsushita, H., Li, W., Glassford, AJ., Asagami, T., Lee, KH., Harrison, DJ., Tsao, PS., (2001), Diabetes Mellitus enhances vascular matrix metalloproteinase activity. Role of oxidative stress, Circulation Research, 88, p1291-1298 .
-
Ugwu, F., Van Hoef, B., Bini, A., Collen, D., Lijnen, HR., (1998), Proteolytic cleavage of urokinase-type plasminogen activator by stromelysin-1 (MMP-3), Biochemistry, 37, p7231-7236 .
[https://doi.org/10.1046/j.1365-2249.1999.00764.x]

- Van der Zee, E., Everts, V., Beertsen, W., (1996), Cytokineinduced endogenous procollagenase stored in the extracellular matrix of soft connective tissue results in a burst of collagen breakdown following its activation, J Periodontal Res, 3, p483-488 .
- Van der Zee, E., Everts, V., Beertsen, W., (1997), Cytokines modulate routes of collagen breakdown, J Clin Periodontology, 24, p297-305 .
- Von Bredow, DC., Cress, AE., Howard, EW., Bowden, GT., Nagle, RB., (1998), Activation of gelatinase-tissueinhibitors-of-metalloproteinase complexes by matrilysin, Biochem J, 331(Pt 3), p965-972 .
- Wassenaar, A., Verschoor, T., Kievits, F., Den Hartog, MT., Kapsenberg, ML., Everts, V., Snigders, A., (1999), CD40 engagement modulates the production of matrix metalloproteinases by gingival fibroblasts, Clin Exp Immunol, 115, p161-167 .
- Weiss, SJ., (1989), Tissue destruction by neutrophils, N Engl J Med, 320, p365-376 .
-
Werb, Z., (1997), ECM and cell surface proteolysis : regulating cellular ecology, Cell, 91, p439-442 .
[https://doi.org/10.1016/S0092-8674(00)80429-8]
