Antimicrobial activity of modified 4-META/MMA-TBB resin filled with pre-polymerized filler particles
In this study, to evaluation the chlorhexidine (CHX) release from the modified 4-META/MMA-TBB (4-META) resin filled with pre-polymerized filler containing human serum albumin (HSA) nanoparticles. The 4-META resin and modified 4-META resin specimens containing the CHX-loaded HSA nanoparticles were prepared as follows manufacturer. The morphology of the resin containing nanoparticles were observed using scanning electron microscopy (SEM) and in vitro release profile of CHX from the resin was measured by placing the samples in phosphate-buffered saline (PBS, pH 7.4) at 37˚C for up to 30 days. The SEM image of the resin clearly shows that the CHX-loaded HSA nanoparticles were homogeneously dispersed and well-incorporated in the two different resin matrix. Moreover, CHX was successfully released from the two different resin matrix for a long period of time (up to 30 days incubation time). However, in comparison with the 4-META resin containing CHX-loaded HSA nanoparticles, the release profile of the modified 4-META resin containing CHX-loaded HSA nanoparticles showed a lower amount of CHX released over the study period. The flexural strength and tensile bond strength remains unchanged by incorporation of CHX-loaded HSA nanoparticles and CHX powder in two different 4-META resin (p > 0.05).
Keywords:
4-META/MMA-TBB resin, antimicrobial, chlorhexidine, pre-polymerized filler, tri-n-butylborane서 론
The mechanical properties and wear resistance of various dental restorative materials have been improved considerably. However, the antimicrobial properties of the dental restorative materials are still limited (Shinohara et al., 2009).
The so-called 4-META resin, which is one of the dental adhesive resin systems, consists of 4-acryloyloxyethyl trimellitate anhydride (4-META), methyl methacrylate (MMA), and tri-n-butylborane (TBB) (4-META/MMA-TBB). This resin can be used for the direct fixation of mobile teeth because of its unique resilience. Morever, the resin is highly biocompatible to tooth pulp tissue (Kudou et al., 2000).
Attrition and wear of enamel and dentine, and wedgeshaped defects gradually progress due to occlusal contact, mastication, grinding, tooth brushing, and other factors. It would likely be beneficial for both tooth structure and dental material of interface integrity and wear resistance were equivalent, particularly within the molar Occlusal plane (Naito, 2011). An adhesive resin chemically initiated with tri-n-butylborane (TBB) has been used for bonding between tooth structure and restorative materials (Hosoya and Tay, 2014)
Recently, a modified 4-META based resin was developed for both luting and restorative agents, and, unlike the original TBB resin, it contains TMPT (trimethylol propane trimethacrylate) pre-polymerized filler particles (Tanimura and Suzuki, 2014). The resin polymerization is initiated by TBB which has been used in 4-META based resins for a long time (Okamoto and Takahata, 1998). This material shows an excellent bonding effect to teeth, ceramics, and metals when apposite primers are used (Nukui and Yorozhu, 2011). It also presents higher wear resistance compared with the conventional 4-META resin (Naito, 2011).
However, 4-META resin and modified 4-METEA resin lacks antimicrobial activity against oral bacteria. Therefore, the incorporation of antibacterial agents into this resin would help provide therapeutic effects. A previous study suggested that the incorporation of Chlorhexidine (CHX)-loaded human serum albumin (HSA) nanoparticles into the 4-META resin is optimal in terms of the antibacterial effects and mechanical properties (flexural and shear bond strength). In that study, CHX-loaded HSA nanoparticles was incorporated directly into the monomer and long-term CHX release was observed (Kim et al., 2014). Like 4-META resin, modified 4-META resin lacks antimicrobial activity against oral bacteria. However, to the best of the authors’ knowledge, there are no reports on the application of antimicrobial agents to introduce the antimicrobial effect of modified 4-META resin.
In the present study, a functional modified 4-META adhesive with an antibacterial effect was developed using CHX-loaded HSA nanoparticles to control the release of CHX from the modified 4-META resin. The purpose of this study was to evaluate the antimicrobial effect and mechanical properties of 4-META resin filled with pre-polymerized composite particles compare with conventional 4-META resin.
MATERIALS AND METHODS
The resins compositions are listed in Table 1. Two different types of 4-META/MMA-TBB resins tested in this study were: 4-META/MMA-TBB resin (SB, Super bond C&B, Sun Medical Co. Ltd., Kyoto, Japan) and modified 4-META/MMA-TBB resin containing prepolymerized filler particles (BF, Bondfill SB, Sun Medical Co. Ltd.) (Kitasako et al., 2002).
Fabrication of CHX-loaded nanoparticles and resin specimens
CHX-loaded HSA nanoparticles were prepared by a desolvation method as described previous study (Kim et al., 2014). Briefly, 100 mg of HSA (Sigma Co., USA) and 20 mg of CHX diacetate (Sigma Co.) were prepared in 10 mL of distilled water and transformed to nanoparticles by the continuous addition of 10 mL of the desolvating agent (acetone) with constant stirring at room temperature. Subsequently, a glutaraldehyde solution (0.25 %) was added to induce particle crosslinking. The nanoparticles were then freeze-dried.
The compositions of the experimental specimens are listed in Table 2. Each 4-META resin and modified 4-META specimens were prepared according to the manufacturer’s instructions. The CHX-loaded HSA nanoparticles (1 mg, 0.5 % wt/wt) were dispersed in the resin monomer liquid, and mixed with TBB solution and PMMA powder. Two resin paste containing the CHX power and CHX-loaded HSA nanoparticles was placed into a mold (8 mm in diameter, 1 mm thickness) and allowed to set at room temperature for 24 h. The control specimen was prepared without nanoparticles using the same procedure described above, respectively. The morphology of the distribution of CHX-loaded HSA nanoparticles in the two different resin were examined using scanning electron microscopy (SEM, JSM-6700F, Jeol, Japan).
Mechanical test(Flexural strength and tensile bond strength)
Flexural strength test specimens, according to ISO 4049, the resins were inserted in a rectangular stainlesssteel mold with 2 mm × 2 mm × 25 mm in size, which was placed on a glass slide. Then, the mold was covered with another glass slide. The specimens were removed from the mold and stored in distilled water for 24 h at 37°C prior to the test. Both surfaces of all specimens were polished using a 600 grit silicon carbide paper in a moist environment. At least 10 specimens were tested for each formulation. A three-point bending test was performed using a universal testing machine (3366, Instron Inc., USA) at a cross-head speed of 1 mm/min.
To define the bonding area, a piece of 50 μm-thick masking tape with a circular hole (4 mm in diameter) was placed onto each polished (to the final level of a 2,000 grit silicon carbide paper) titanium (JIS Class 2, Toho Titanium Co., Japan) disc (Kwon et al., 2010; Kim et al., 2014). Mixed two different 4-META resin with or without the CHX-loaded HSA nanoparticles were applied to the bonding surface and affixed to an acrylic rod. The bonded specimens were left undisturbed for 30 min in air at room temperature, and immersed in water at 37°C for overnight. The tensile bond strength of each specimen was measured using a universal testing machine at a crosshead speed of 1 mm/min.
In vitro release profile of CHX
The release profiles of CHX were examined in two different resin matrix containing CHX-loaded HSA nanoparticles. The disc-shaped 4-META resin and modified 4-META resin specimens containing the CHX-loaded HSA nanoparticles were immersed in 3 mL of phosphate buffer saline (PBS) (pH 7.4) and stored at 37°C for up to 30 days under static conditions. Aliquots of the PBS solution were collected at predetermined times (1, 3, 5, 10, 15, 20, 30 days), and the same volumes (1.5 mL) of fresh PBS were added. The concentration of CHX released was determined by high performance liquid chromatography (HPLC, LC-20AD, Shimadzu Corp., Japan). Values are reported as the mean±SD of the five replicates. The mobile phase was composed of 70 % v/v HPLC grade acetonitrile and 30 % v/v HPLC grade water. The flow rate was 1.0 mL/min and the components were detected at 254 nm. The encapsulation efficiency was then calculated using the amount of CHX loaded into the nanoparticles: amount of CHX loaded/ theoretical maximum drug loading (Sebak et al., 2010).
RESULTS AND DISCUSSION
Morphological characteristics of the specimens
Chlorhexidine is the most popular antiseptic of biguanides. It has potent antimicrobial activity against most Gram-positive and some Gram-negative bacteria but not against spores (Havlíkováet al., 2007). However, the release of antimicrobial agents from the material into the surrounding milieu has several common problems: decreased mechanical properties of the carrier material, short-term antimicrobial effectiveness (Beyth et al., 2006). For the reason, in this study, 4-META resin with antimicrobial properties was used nanoparticles as a drug delivery carrier system. The advantages of using nanoparticles as a drug delivery system controlled release and particles degradation characteristics can be readily modulated by choice of matrix constituents (Mohanraj and Chen, 2006).
Figure 1 shows cross-section SEM images of the two different 4-META resin specimens with and without the CHX-loaded HSA nanoparticles. Figure 1(A, B) shows dense and compact matrix morphologies of the two different 4-META resin. Fig. 1(A1, B1) clearly shows that the CHX-loaded HSA nanoparticles were distributed homogeneously over the two resin matrix. These results indicated that CHX-loaded HSA nanoparticles were successfully embedded in the two 4-META resin matrix. The functional monomer 4-META in MMA consists of hydrophobic and hydrophilic groups, which makes the MMA slightly hydrophilic (Imai et al., 1991). Therefore, a small amount of hydrophilic HSA albumin nanoparticles can be miscible with the MMA containing two different 4-META resin (Kwon et al., 2010).
Figure 2 shows that the incorporation of the CHXloaded HSA nanoparticles did not significantly affect the flexural and tensile bond strength of the two different 4-META resin (one-way ANOVA, p > 0.05).
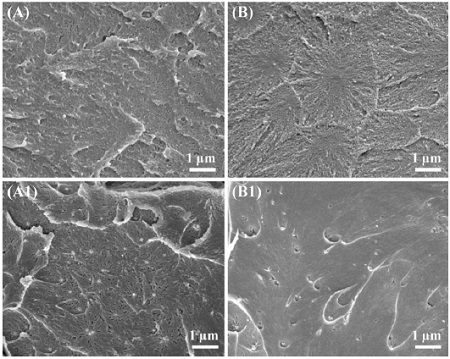
SEM image of 4-META resin (A), 4-META resin containing CHX-loaded HSA nanoparticles (A1), modified 4-META resin (B) and modified 4-META resin containing CHX-loaded HSA nanoparticles (B1). (Original magnification 10,000×).
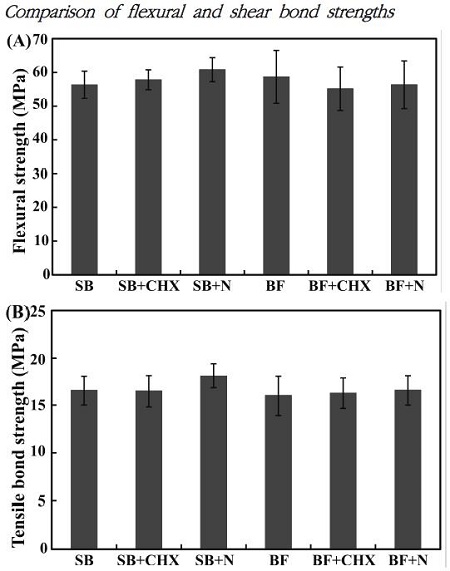
Flexural strength results for the six groups (A) and tensile bond strength results for the six groups (B) (n=10).
In this study, two types of 4-META/MMA-TBB resin cements were investigated. Because 4-META resin contains no filler, mechanical failure can occur more easily leading to restoration failure. The modified 4-META resin has an pre-polymerized filler (TMPT) content of 5%. This filled TMPT pre-polymerized filler 4-META resin cement also showed high tensile and shear dentin bond strengths at 24 h after cementation (Kitasako et al., 2002). The difference bond strength between two type 4-META resins was not significant when resin matrix containing CHX-loaded HSA nanoparticles, possible because the composition of these resins is almost identical. The present results also suggest that incorporation of pre-polymerized fillers does not affect the mechanical performance of 4-META resin (Naito, 2011).
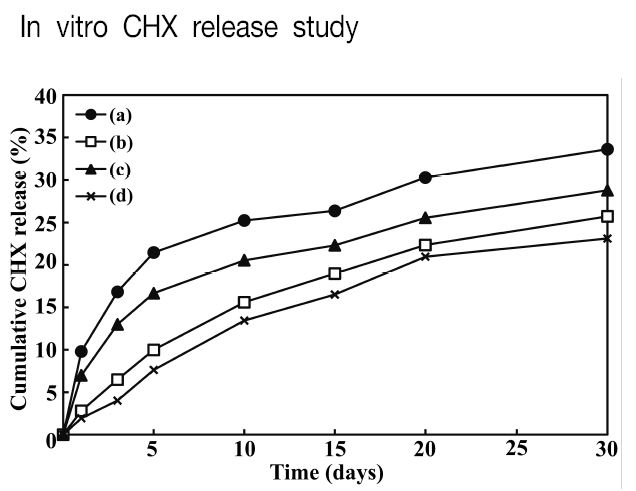
In vitro CHX release study
Figure 3 shows the in vitro CHX release profile of two different 4-META resin containing CHX-loaded HSA nanoparticles. The CHX was successfully released from the two resin matrix for a long period of time (up to 30 days incubation time). However, in comparison with the 4-META resin, the release profile of the modified 4-META resin captaining CHX-loaded HSA nanoparticles showed a lower amount of CHX released over the study period. Conventional 4-META resin is mainly composed of acrylic resin (MMA and PMMA). In contrast, modified 4-META resin is mainly composed of a acrylic resin added small quantity pre-polymerized filler. Monomer diffusion and polymerization of 4-META/MMA-TBB resin are competitive (Nakabayashi et al., 1982).
This release behavior was attributed to the 4-META resin that delayed contact with water to the nanoparticles. This allowed the slow release of CHX through the nanovoids in the resin matrix in same manner as the control HSA nanoparticles. A previous study confirmed that the incorporation of 1.0-1.5 % chlorhexidine (CHX) digluconate into the 4-META resin is antibacterial effects. In that study, CHX digluconate liquid was incorporated only short-term release was observed (Kwon et al., 2010). Therefore, the HSA nanoparticle-containing two different 4-META resin could be used effectively for the long-term sustained release of antimicrobial molecules at an adhesive site.
In the present study, the antimicrobial effect of CHX-loaded HSA nanoparticles to the materials was not observed, our focus being only on mechanical properties between the 4-META and modified 4-META resin. Therefore, further investigation using for agar diffusion test is required to clarify the relationship between the antimicrobial effects of two 4-META resin containing CHX-loaded HSA nanoparticles to streptococcus mutans.
CONCLUSIONS
The CHX-loaded HSA nanoparticles were distributed homogeneously over the 4-META resin and modified 4-META resin matrix filled with pre-polymerized filler, which did not affect the flexural and adhesion properties of the titanium. The CHX released from the two different 4-META resin matrix was controlled efficiently over the experimental period through the use of CHX-loaded HSA nanoparticles. However, in comparison with the 4-META resin, the release profile of the modified 4-META resin captaining CHX powder and CHX-loaded HSA nanoparticles showed a lower amount of CHX released.
References
-
Beyth, N., Yudovin-Farber, I., Bahir, R., Domb, AJ., Weiss, EI., (2006), Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans, Biomaterials, 27, p3995-4002.
[https://doi.org/10.1016/j.biomaterials.2006.03.003]

- Havlíáková, L., Matysová, L., Nováková, L., Hájková, R., Solich, P., (2007), HPLC determination of chlorhexidine gluconate and p-chloroaniline in topical ointment, J Pharm Biomed Anal, 43, p1169-1173.
-
Hosoya, Y., Tay, FR., (2014), Bonding ability of 4-META self-etching primer used with 4-META/MMA-TBB resin to enamel and dentine: Primary vs permanent teeth, J Dent, 42, p425-431.
[https://doi.org/10.1016/j.jdent.2014.01.007]

-
Kim, HJ., Kwon, TY., Kim, KH., Kwon, ST., Cho, DH., Son, JS., (2014), Long-term release of chlorhexidine from dental adhesive resin system using human serum albumin nanoparticles, Polym Bull, 71, p875-886.
[https://doi.org/10.1007/s00289-014-1099-0]

-
Kitasako, Y., Burrow, MF., Nikaido, T., Tagami, J., (2002), Long-term tensile bond durability of two different 4-META containing resin cements to dentin, Dent Mater, 18, p276-280.
[https://doi.org/10.1016/S0109-5641(01)00049-5]

-
Kudou, Y., Obara, K., Kawashima, T., Kubota, M., Abe, S., Endo, T., Komatsu, M., Okuda, R., (2000), Addition of antibacterial agents to MMA-TBB dentin bonding systems-influence on tensile bond strength and antibacterial effect, Dent Mater J, 19, p65-74.
[https://doi.org/10.4012/dmj.19.65]

-
Kwon, TY., Hong, SH., Kim, YK., Kim, KH., (2010), Antibacterial effects of 4-META/MMA-TBB resin containing chlorhexidine, J Biomed Mater Res B Appl Biomater, 92, p561-567.
[https://doi.org/10.1002/jbm.b.31553]

-
Mohanraj, VJ., Chen, Y., (2006), Nanoparticles-a review, Trop J Pharm Res, 5, p561-573.
[https://doi.org/10.4314/tjpr.v5i1.14634]

-
Naito, K., (2011), Bonding and wear characteristics of a tri-n-butylborane initiated adhesive resin filled with pre-polymerized composite particles, J Oral Sci, 53, p109-116.
[https://doi.org/10.2334/josnusd.53.109]

-
Nakabayashi, N., Kojima, K., Masuhara, E., (1982), The promotion of adhesion by the infiltration of monomers into tooth substrates, J Biomed Mater Res, 16, p265-273.
[https://doi.org/10.1002/jbm.820160307]

- Nukui, Y., Yorozhu, K., (2011), Introduction of bondfill SB, Dent Mag, 136, p44-46.
- Okamoto, Y., Takahata, K., (1998), Studies on the behavior of partially oxi-dized tributylborane as a radical initiator for methyl methacrylate (MMA) polymerization, Chem Lett, 1998, p1247-1248.
-
Imai, Y., Kadoma, Y., Kojima, K., Akimoto, T., Ikakura, K., Ohta, T., (1991), Importance of polymerization initiator systems and interfacial initiation of polymerization in adhesive bonding of resin to dentin, J Dent Res, 70, p1088-1091.
[https://doi.org/10.1177/00220345910700071401]

- Sebak, S., Mirzaei, M., Malhotra, M., Kulamarva, A., Prakash, S., (2010), Human serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: preparation and in vitro analysis, Int J Nanomedicine, 20, p525-532.
-
Shinohara, MS., De Goes, MF., Schneider, LF., Ferracane, JL., Pereira, PN., Di Hipólito, V., Nikaido, T., (2009), Fluoride-containing adhesive: durability on dentin bonding, Dent Mater, 25, p1383-1391.
[https://doi.org/10.1016/j.dental.2009.06.011]

- Tanimura, R., Suzuki, S., (2014), In vitro evaluation of a modified 4-META/MMA-TBB resin for filling access holes of screw-retained implant prostheses, J Biomed Mater Res B Appl Biomater.