Photocatalytic effect of doped-TiO2 nanoparticles on discolored teeth
Abstract
The aim of this study was to determine the photocatalytic effect of doped-TiO2 nanoparticles (NPs) on teeth bleaching with an aid of 3% H2O2 and laser irradiation. For the study, Mo-N-TiO2 NPs were prepared. The characteristics of the prepared NPs, NPs morphology and light absorbance, were evaluated. Photocatalytic reactions of NPs were tested using 10 ppm methylene blue (MB) solution. Extracted teeth were pasted using carbomer gel for color differences measurements. Mo-N-TiO2 NPs have close to round shape with some tens nm size. Their absorbance was higher and longer than that of TiO2 NPs. For MB solution, Mo-N-TiO2 with 3% H2O2 condition showed much decrease in absorbance after laser irradiation for 20 min. Also, regardless of wavelength, Mo-N-TiO2 NPs produced much greater color difference (whitening) on teeth after 3 h than that by 15% H2O2.
초록
본 연구는 3% H2O2와 레이저 광조사하에서 도핑된 TiO2의 변색된 치아에 대한 광촉매 효과를 평가한 것이다. 이를 위하여 Mo-N-TiO2 나노입자를 제조하고 입자의 형상과 광흡수도를 평가하였다. 액상의 Methylene blue(MB)에 대한 광촉매 효과와 카보머를 사용하여 발거된 치아에서의 색변화를 측정하였다. Mo-N-TiO2는 크기가 수십 nm이고 원형에 가까웠으며 광흡수도는 TiO2의 그것보다 크고 길었다. MB 용액에서 3% H2O2를 적용한 Mo-N-TiO2는 20분 동안 레이저로 광조사 하였을 때 흡수도가 크게 감소하였다 (즉 탈색되었다). 또한 입사광의 파장에 상관없이 Mo-N-TiO2는 3시간 후에 15% H2O2 의한 것 보다 큰 치아의 색변화 (미백을)를 보였다.
Keywords:
Bleaching agent, Mo-N-doped TiO2, Absorbance, Photocatalytic effect, Color difference키워드:
미백재, 흡수도, 광촉매 효과, 색변화Introduction
Everybody wishes to have clean and white teeth. However, despite these small wishes, keeping the teeth white through the daily routine brushing is not easy because of many factors around us those are beyond our control. Basically, teeth discoloration is the result of the change of tooth color, hue, and translucency. In most cases, to be discolored, any source of stains should be adsorbed on the tooth surface or absorbed into the tooth subsurface (1-4). Generally, the most easily encountered stain sources are coming from foods, beverages, and smoking. If stains from these sources are not regularly and completely removed from the tooth surface, discoloration will be gradually developed. Tooth brushing is one easy option for removing stains from the tooth surface. However, stains in the tooth subsurface are not readily removed by brushing unless the subsurface is not fully worn out.
Peroxide-based bleaching agents are materials that whiten the discolored teeth with simple and easy manner. Hydrogen peroxide is a bleaching agent that is used at home and clinics with 3~35% concentration. Carbamide peroxide (CH6N2O3) is another choice. Carbamide peroxide usually decomposes into urea (CH4N2O) and hydrogen peroxide (H2O2). The concentration of 10% carbamide peroxide is equivalent to 3% H2O2. Hydrogen peroxide can readily form free radicals by heat, light, or catalyst (5,6). Also, through the Fenton reaction with metal ions, H2O2 can form free radicals. Such formed free radicals can penetrate the teeth subsurface and decompose stains (7, 8).
To enhance bleaching efficiency by free radicals, titanium dioxide (TiO2) was added in the bleaching agent. Added TiO2 can produce free radicals, such as hydroxyl (OH•) and superoxide anion (O2•-), through the photocatalytic reaction by external light using water and oxygen (9-11). However, since TiO2 absorbs light only in the UV range, it needs to extend the absorption to the visible range. To handle the problem, metal ion or gas was doped into the TiO2 and the absorbance of doped-TiO2 had extended to the visible range (12-14).
Using lower concentration (3%) H2O2 to achieve acceptable level of bleaching requires long treatment time (2-4 weeks with daily 6-8 h treatment), though this treatment minimizes tooth damage. The purpose of the present study was to evaluate teeth bleaching using doped-TiO2 nanoparticles (NPs) and 3% H2O2 under laser irradiation. Through the study, the photocatalytic reactions of doped-TiO2 induced by visible light were tested in terms of teeth whiteness.
Materials and Methods
1. Synthesis of Mo-N-doped TiO2 nanoparticles (NPs)
For the study, Mo-N-TiO2 NPs were synthesized using ammonium molybdate tetrahydrate [(NH4)6Mo7O24·4H2O] as a precursor of Mo and N. 1 g cetyl trimethyl ammonium bromid (CTAB) was dissolved in a mixture of 10 mL tetrabutyl titanate (TTOB), 80 mL ethanol, and 6 mL nitric acid (70%) to make a homogeneous solution. Then 18 ml (NH4)6Mo7O24·4H2O solution (0.05 M) was added dropwise under strong stirring. The obtained sol was kept in oven for 48 h at 50℃ to get the gel. The obtained gel was calcined at 500℃ for 4 h to get the Mo, N co-doped TiO2, Mo-N-TiO2 (15). All chemicals were analytical reagent grade, purchased from Sigma Aldrich (St. Louis, MO, USA), and used without additional purification.
2. Characterization and photocatalytic reaction of NPs
To characterize Mo-N-TiO2 NPs, the particle morphology of NPS was observed using TEM at high magnification (×200k). The absorbance of NPs was measured by UV-VIS spectrophotometer (Jasco V-670, Jasco, Tokyo, Japan), using BaSO4 as the reference. The photocatalytic performance (bleaching) of the prepared Mo-N-TiO2 NPs was tested using methylene blue (MB) and the extracted teeth. The test conditions are shown in Table 1.

Tested photocatalytic conditions for degradation of MB solution and bleaching of extracted caries-free teeth
To perform the MB test, 4 mL MB solution (10 ppm) was mixed with each NP (1 wt%) in a 5 mL beaker and stirred using a magnetic bar during light irradiation. To irradiate light, lasers of different wavelengths (405, 660 nm; LVI Technology Inc., Yongin, Korea) were irradiated under 50 mW/cm2 condition for 20 min. Degradation by photocatalytic effect was measured using a spectrophotometer (SpectraMax 190, Molecular Devices, San Jose, CA, USA) at the absorbance mode.
The efficiency of bleaching (photocatalytic performance) of Mo-N-TiO2 NPs for different test conditions was tested using the caries-free extracted teeth. Teeth (n=5 for each condition) were cleaned, frozen, and stored at a relative humidity of 100% after extraction. The Institutional Review Board at Pusan National University Dental Hospital, Yangsan, Korea, approved the study and waived informed consent. Before tests, teeth were brushed, sonicated, and dried. For consistency and convenience of the measurements, specimens were placed over putty in a petri dish (radius: 60 mm, height: 20 mm) along the imaginary circle. During the experiment, each specimen on the putty, and petri dish on the table, were placed at a pre-fixed position.
The first color measurements of the specimen teeth were performed before bleaching gel treatment using a digital spectrometer (Easyshade V, Vita Zahnfabrik, Bad Säckingen, Germany). At first, the device was set up to basic shade measurement mode and then the probe tip was positioned in close contact with the specimen surface. The measurements were performed three times at the same fixed position. After the initial color measurement, all teeth surfaces were pasted with carbomer gel, which containing H2O2, Mo-N-TiO2 NPs, H2O2+Mo-N-TiO2 NPs, to 1 mm thick, followed by laser irradiation using the same light conditions stated above, for 3 h. After 3 h, all cabomer gel over the specimens was carefully removed, cleaned using tap water, and remaining moisture on the surface was cleaned using soft tissue. The petri dish was exactly placed at the initial position, and the second color measurement was performed. Using the color values (L*, a*, b*) obtained, the color difference was calculated as follows:
where ΔL* , Δa* , and Δb* are the changes in L*, a* , and b*, respectively; L* represents the degree of grayness and corresponds to lightness. The parameter a* represents the red (for +a* value) - green (for -a* value) axis, whereas b* is a parameter in the blue (for -b* value) - yellow (for +b* value) axis.
3. Statistical analysis
The result of color difference was analyzed by one-way ANOVA followed by a Tukey’s post-hoc test for multiple comparisons; p values <0.05 were considered significant.
Results
Figure 1 shows the TEM image of the tested NPs. Particles show close to the round shape with some tens nm size.
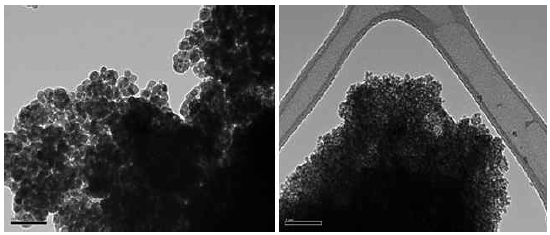
TEM image of the tested NPs. Scale bar in the left and right figures represents 100 nm and 1 μm, respectively.
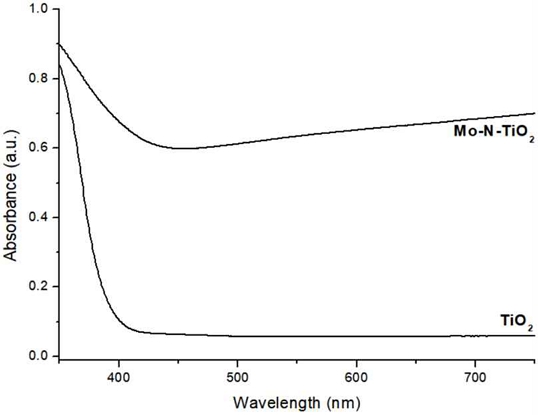
Figure 2 shows the absorbance of TiO2 and Mo-N-TiO2. Unlike TiO2, Mo-N-TiO2 shows high and steadily increasing absorbance after 400 nm.
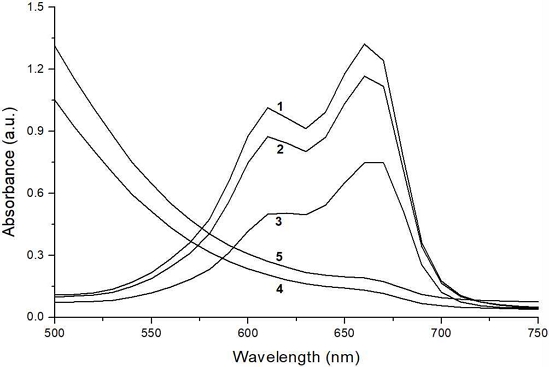
Figure 3 shows the degradation (bleaching) of MB solution by different test conditions (1: MB 10 ppm, 2: MB+3% H2O2, 3: MB+15% H2O2, 4: MB+Mo-N-TiO2+3% H2O2+405 nm laser, 5: MB+Mo-N-TiO2+3% H2O2+660 nm laser). For conditions 2 and 3, H2O2 was treated for 30 min, and for conditions 4 and 5, laser was irradiated for 20 min. As a result, laser-treated specimens showed much degradation of MB solution than those treated only with H2O2 even though laser was irradiated with much less time.
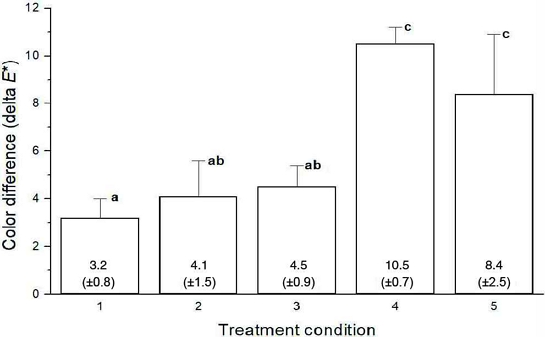
Figure 4 shows the resultant color difference (ΔE*) of the discolored teeth by different test conditions. The laser-treated specimens regardless of light wavelength showed much and significantly different bleaching (whitening) (p<0.05) than those treated only with H2O2.
Discussion
Teeth bleaching (whitening) is a process that makes teeth white through bleaching the discolored teeth using a bleaching agent. Though it is not a dental hygiene issue and essential for dental health, it attracts much attention from people because everybody wishes clean and white teeth regardless of gender and generation. Peroxide-based agents, such as carbamide peroxide and hydrogen peroxide, are basic tooth whitening agent. They can be used at home and dental clinics depending on the concentration of agent. Hydrogen peroxide of 3% (or 10% carbamide peroxide) can be easily and safely used at home without a guidance of dental specialist. It usually takes long treatment time, 2-4 weeks, to achieve some level of whiteness. So, there are much need to shorten the treatment time without lessening the level of whiteness.
Bleaching of the discolored teeth, though the mechanism is not fully understood yet, occurs by cleaving the long chain molecules that cause teeth discoloration into short chain molecules by reactive radicals which are produced from the peroxide-based bleaching agent (2, 4). Photocatalytic reaction (photolysis) is a process that accelerates the photoreaction in the presence of catalyst. So to initiate the process, both a proper wavelength of light and catalyst are essential. Titanium dioxide (TiO2) is a well-known catalyst. The process takes place in a way that the light-absorbed TiO2 creates hole-electron pairs in their valence band (VB) and conduction band (CB). The formed hole (h+) in the VB and transferred electron (e-) from VB into CB interacts with neighbor water and oxygen and then produce hydroxyl radical (OH•) and superoxide anion radical (O2•-) depending on the neighbor environment. To initiate photocatalytic process with TiO2, at least 3.2 eV or more photon energy is required to conduct electron transition, it means the use of hazardous UV as a light source is inevitable.
The way to avoid this issue is using a visible light whose wavelength is longer than 400 nm, the maximum absorbance band of TiO2. By doping (gas or metal) ions into TiO2 as an impurity, doped-TiO2 can absorb light longer than 400 nm (12-14). By doing so, visible light can be used as an alternative of UV light. In the present study, Mo-N-TiO2 NPs was tested. This NPs showed much higher and longer absorption band than that of TiO2 after 400 nm. According to results, MB solutions treated with Mo-N-TiO2 NPs showed much enhanced degradation with an aid of visible light. Furthermore, laser (light) and TiO2-treated MB solutions with an aid of 3% H2O2 showed much greater degradation than that by 15% H2O2, the highest concentration allowed in Korea for the specialist use. The reason for this difference would be the difference of the amount of the formed free radicals. Hydrogen peroxide treated with doped-TiO2 under external light may produce much free radicals through the photocatalytic reaction by breaking oxygen-rich H2O2 if it is present nearby.
To speed up the bleaching, high concentration H2O2 is a primary choice up to 35%. However, though it is good to reduce the total length of treatment time, it usually accompanies some loss of minerals, dehydration of teeth, weakness of surface hardness, and poking pain (15-17). Practically, achieving high bleaching with less affected (damaged) teeth seems not achievable with conventional approaches. However, within a same length of time (3 h), the teeth treated with 3% H2O2+Mo-N-TiO2 NPs+laser conditions achieved much higher color difference (teeth whitening) compared to that by 15% H2O2 (4.5 vs. 10.5 or 8.4) regardless of light wavelength. Treatment for 3 h with H2O2 is close to the total accumulation time for 15% H2O2 treatment in the dental clinic. Furthermore, in case of 3% H2O2, it is much less time than that recommended for one-day treatment for home bleaching. It is usually recommended overnight treatment/day for 7 days with 2-4 week procedures. However, despite of the achieved progress in reducing treatment time per day, a further tests are suggested by increasing the number of specimens to confirm the effectiveness of the tested specimens.
Conclusions
Within the limitations of the present study, the following conclusions could be reached:
- 1. Mo-N-TiO2 NPs showed high color difference (up to 10.5) in the discolored teeth if combined with 3% H2O2 and visible laser irradiation for 3 hr. However, 158 15% H2O2 case showed only 4.5.
- 2. Two lasers (405 and 660 nm) achieved higher color difference compared to that by 15% H2O2. Between two lasers, 405 nm laser achieved higher bleaching (color difference) than that by 660 nm laser.
Acknowledgments
This work was supported by a 2-Year Research Grant of Pusan National University
References
-
Epple M, Meyer F, Enax J. A critical review of modern concepts for teeth whitening. Dent J (Basel). 2019;7(3):E79.
[https://doi.org/10.3390/dj7030079]

-
Clifton M, Carey J. Tooth Whitening: What We Now Know. Evid Based Dent Pract. 2014;14(Supplement): 70-6.
[https://doi.org/10.1016/j.jebdp.2014.02.006]

-
Joiner A, Luo W. Tooth colour and whiteness: A review. J Dent. 2017;67S:S3-S10.
[https://doi.org/10.1016/j.jdent.2017.09.006]

-
Kwon SR, Wertz PW. Review of the Mechanism of Tooth Whitening. J Esthet Restor Dent. 2015;27(5):240-57.
[https://doi.org/10.1111/jerd.12152]

-
Buchalla W, Attin T. External bleaching therapy with activation by heat, light or laser-a systematic review. Dent Mater. 2007;23(5):586-96.
[https://doi.org/10.1016/j.dental.2006.03.018]

-
Yu H, Li Q, Wang YN, Cheng H. Effects of temperature and in-office bleaching agents on surface and subsurface properties of aesthetic restorative materials. J Dent. 2013;41(12):1290-6.
[https://doi.org/10.1016/j.jdent.2013.07.015]

-
Kim Y, Son HH, Yi K, Ahn JS, Chang J. Bleaching Effects on Color, Chemical, and Mechanical Properties of White Spot Lesions. Oper Dent. 2016;41(3):318-26.
[https://doi.org/10.2341/15-015-L]

-
de Araújo LS, dos Santos PH, Anchieta RB, Catelan A, Fraga Briso AL, Fraga Zaze AC, Sundfeld RH. Mineral loss and color change of enamel after bleaching and staining solutions combination. J Biomed Opt. 2013;18(10):108004.
[https://doi.org/10.1117/1.JBO.18.10.108004]

-
Poulopoulos SG, Yerkinova A, Ulykbanova G, Inglezakis VJ. Photocatalytic treatment of organic pollutants in a synthetic wastewater using UV light and combinations of TiO2, H2O2 and Fe(III). PLoS One. 2019;14(5):e0216745.
[https://doi.org/10.1371/journal.pone.0216745]

-
Kazuya N, Akira F. TiO2 photocatalysis: Design and applications. J Photoch Photobio C. 2012;13(3):169-89.
[https://doi.org/10.1016/j.jphotochemrev.2012.06.001]

-
Etacheri V, Di Valentinc C, Schneider J, Bahnemann DW, Pillaifg SC. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J Photoch Photobio C. 2015;25:1-29.
[https://doi.org/10.1016/j.jphotochemrev.2015.08.003]

-
Wang S, Xu J, Ding H, Pan S, Zhang Y, Li G. Facile synthesis of nitrogen self-doped rutile TiO2 nanorods. CrystEngComm. 2012;14:7672–9.
[https://doi.org/10.1039/c2ce25827g]

-
Leonga KH, Bee Gana L, Ibrahima S, Saravanan P. Synthesis of surface plasmon resonance (SPR) triggered Ag/TiO2 photocatalyst for degradation of endocrine disturbing compounds. Appl Surface Sci. 2014;319:128–35.
[https://doi.org/10.1016/j.apsusc.2014.06.153]

-
Hu HZ, Li FY, Fan ZP. Enhanced Visible Light Activity and Stability of TiO2 Nanopowder by co-doped with Mo and N. Bull Korean Chem Soc. 2012;33(4):1269-74.
[https://doi.org/10.5012/bkcs.2012.33.4.1269]

-
Attin T, Schmidlin PR, Wegehaupt F, Wiegand A. Influence of study design on the impact of bleaching agents on dental enamel microhardness: a review. Dent Mater. 2009;25(2):143-57.
[https://doi.org/10.1016/j.dental.2008.05.010]

-
Joiner A. Review of the effects of peroxide on enamel and dentine properties. J Dent. 2007;35(12):889-96.
[https://doi.org/10.1016/j.jdent.2007.09.008]

-
Lee KH, Kim HI, Kim KH, Kwon YH. Mineral loss from bovine enamel by a 30% hydrogen peroxide solution. J Oral Rehabil. 2006;33(3):229-33.
[https://doi.org/10.1111/j.1365-2842.2004.01360.x]