Changes in adhesive strength and pH of dental universal adhesive in accordance with varying proportions of 10-MDP
Abstract
This study investigated the physical properties of dental universal adhesive according to the varying proportions of 10-MDP, a functional monomer that is a representative component of universal adhesives.
Experimental dental universal adhesives were made with 5 different proportions of 10-MDP (from 8.906 wt% to 10.500 wt%). Shear bond strength between bovine teeth and composite resin blocks were measured by applying each experimental adhesive between them. Following the shear bond strength test, fractured surfaces were observed for measurement of the fracture modes. Finally, the pH of the liquid experimental adhesives were measured, to understand results from shear bond strength.
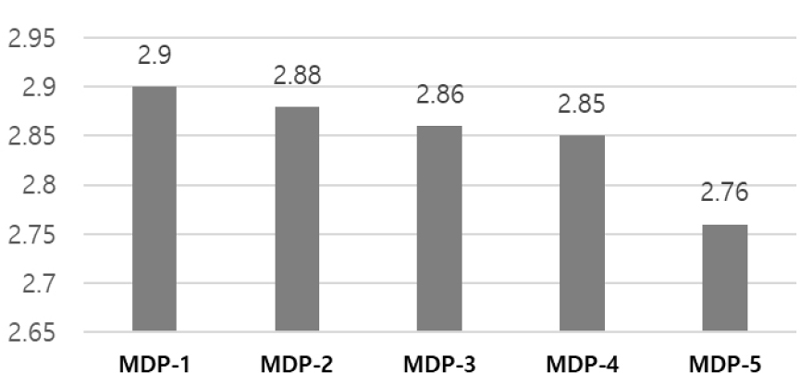
As the results, bonding strength of the experimental dental universal adhesive increased gradually with increasing amounts of 10-MDP, but showed a tendency to decrease gradually after a certain amount was exceeded. The highest adhesive strength was observed when 9.710 wt% of 10-MDP was added. In terms of pH, results showed that as the amount of 10-MDP in the experimental dental universal adhesive increased, the pH decreased from pH 2.9 to pH 2.76.
Based on the results of this study, it was evident that the proportions of 10-MDP resulted in changes in adhesive strength and pH, though the optimal amount of 10-MDP would need a further investigations in the future.
초록
본 연구에서는 치과용 범용 접착제의 대표적인 구성성분인 기능성 모노머 10-MDP의 비율 변화에 따른 치과용 범용접착제의 물리적 성질을 조사하였다.
실험을 위한 치과용 범용 접착제는 5가지 다른 비율의 10-MDP (8.906 wt% ~ 10.500 wt%)로 만들어졌다. 우치와 복합 레진 블록 사이에 각 각의 치과용 범용 접착제를 도포하고 전단결합강도를 측정하였다. 전단결합강도 시험 후, 파괴형태 측정을 위해 파괴된 표면을 관찰하였다. 마지막으로 전단 결합 강도의 결과를 이해하기 위해 액체 상태의 치과용 범용 접착제의 pH를 측정하였다.
그 결과, 치과용 범용접착제의 접착력은 10-MDP의 첨가량이 증가함에 따라 점차 증가하였으나, 일정량 초과 이후에는 점차 감소하는 경향을 보였다. 그 중, 10-MDP를 9.710 wt% 첨가하였을 때 접착력이 가장 높았다. pH 측면에서는 치과용 범용접착제의 10-MDP 함량이 증가할수록 pH가 pH 2.9에서 pH 2.76으로 감소하는 것으로 나타났다.
본 연구 결과를 토대로 10-MDP의 비율이 접착력과 pH에 변화를 가져온다는 것이 분명해졌지만, 10-MDP의 최적량은 향후 추가 조사가 필요하다.
Keywords:
10-MDP, Universal adhesive, Shear bond strength, pH, Dentin adhesive키워드:
범용 접착제, 전단결합강도, 상아질 접착제Introduction
Dental universal adhesive, also known as universal dental adhesive or all-in-one adhesive, is a type of dental adhesive used in restorative dentistry which are named as they are formulated to be compatible with both etch-and-rinse and self-etch techniques (1). Many of dental universal adhesives contain 10-MDP (10-methacryloyloxydecyl dihydrogen phosphate) as the main functional monomer (2). It is a phosphate-containing monomer that helps to create a strong chemical bond between the adhesive and the tooth structure as well as materials such as resin and zirconia (3).
10-MDP works by forming a stable chemical bond with the calcium ions present in the tooth structure. This creates a strong adhesion by forming nano-layering in adhesive layer that is resistant to degradation and allows for the long-term stability of the restoration (4). However, when phosphate containing monomer such as 10-MDP is reacted with water (solvent contains water), the resultant hydrogen ions may lead to acidic pH (5). Previous study showed that pH below 3.0 may result in incompatibility issue when used with self-cure composite, while lower pH may also leads decalcification of hydroxyapatite (6).
A lower pH of universal adhesive is advantageous for etching dentin and enamel. However, excessively low pH can lead to compatibility issues with self-cure composite that involves peroxide and amine reaction mechanisms (7). This issue arises from the acidic monomers remaining in the oxygen-inhibited layer of the bonding, which transform the self-cure composite's 3-valent amines into 4-valent ones. Previous research has shown that the acidity of the bonding must be above 3.0 to resolve this. Therefore, it is necessary to appropriately control the hydrogen ions in phosphate-based monomers like 10-MDP used in universal (8).
Hence, this study aimed to investigate the physical properties of dental universal adhesive according to the proportions of 10-MDP. Optimal amount of 10-MDP in dental universal adhesive was considered by considering shear bond strength using direct composite resins and bovine teeth. Also, changes in pH with varying proportions of 10-MDP were considered in relation to compatibility with self-cure composite in universal adhesives for use in restorative dentistry.
Materials and Methods
1. Preparations of experimental dental universal adhesives
The materials that were used to fabricate experimental dental universal adhesives in this study were listed in Table 1. Through various previous experiments, adhesive ingredients such as Bis-GMA, HEMA, TEGDMA, Ethanol, Water, CQ, EDMAB, and DPPA were undergone sensitivity tests and set up the optimize volume to maximize bonding reliability.
Based on these preliminary experiments, which showed the highest adhesive strength, the amount of 10-MDP ranging from 9 g to 12 g were incorporated into experimental dental universal adhesives, which resulted in final composition with 10-MDP from 8.906 wt% to 10.500 wt% (Table 2). The experimental groups were named MDP-1, MDP-2, MDP-3, MDP-4 and MDP-5.
2. Shear bond strength test
Shear bond strength test was tested by applying experimental dental universal adhesive between the bovine teeth and the composite resin specimens. For bovine teeth, sound and intact maxillary incisors were selected from slaughtered cattle and cut with a low-speed diamond saw (DIAMO-100S, MTDI Co., Daejeon, Korea) to obtain tooth specimens without any caries. The tooth surface debris and pulp tissue were removed, and the specimens were stored in distilled water. The ivory-exposed tooth specimens were stored at 4 ℃ in distilled water until use. The prepared tooths were then embedded in resin (Vertex Self-Curing, Vertex Dental, Soesterberg, Netherland), and polished with #320 and #600 SiC paper until the surface was uniform. The adhesives were applied on the the prepared specimen surface which is dentin, twice for 10 seconds each, with moisture present. The solvents were evaporated using an air blower for 10 seconds, and the adhesives were polymerized for 10 seconds using light curing unit (NOBLESSE, Max Dental Co., Ltd, Bucheon, Gyeongi-do, Korea). The composite resins (TESCERA-Direct, Amco, Seoul, Korea) were then placed onto the polymerized adhesive using a round mold (2.37 mm in diameter and 2.5 mm in thickness) and a bonding mold insert (BOND MOLD A INSERT, Ultradent, South Jordan, UT, USA). Composite resins were polymerized for 40 seconds by light curing unit, pulled out by hands after curing and stored in distilled water at room temperature for 24 hours.
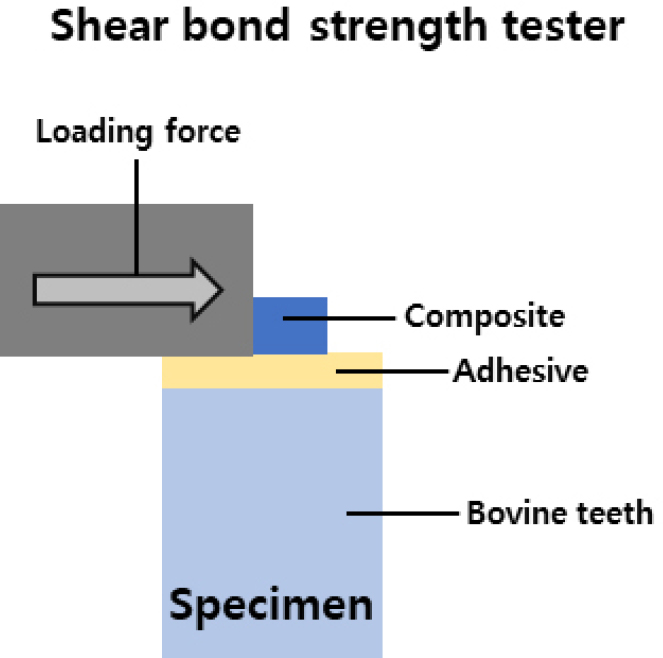
The shear bond strength was then measured using a Shear Bond Tester (T-63010k, Bisco Inc, Schaumburg, IL, USA) with the configurations of the test equipment and the specimen represented as Figure 1. The shear bond strength was then calculated with the equation below;
Following the fracture from the shear bond strength test, the occurrence of cohesive and adhesive fracture was observed.
3. pH measurements of experimental dental universal adhesives
pH meter (Milwaukee MW101 PRO pH Meter, Milwaukee Tool, Brookfield, WI, USA) was used to measure the acidity of liquid adhesive after calibration with buffer solution with pH 4.0 and 7.0 at room temperature. Measurements were carried out 3 times and same number showed in each experimental adhesive.
4. Statistical analysis
The statistical significances of the resulting data were analyzed using one-way ANOVA. The statistical significance was accepted at confidence level of 95 % (p < 0.05) by Tukey’s test for a multiple comparison procedure. The SPSS program (IBM, Amonk, NY, USA) was used for the statistical analysis.
Results
1. Shear bond strength
The results of shear bond strength test are summarized in Table 3. The experimental results showed that there was a significant difference between all groups (p < 0.05) except between MDP-4 and MDP-5 (p > 0.05). As the amount of 10-MDP increased, the shear bond strength initially increased and then decreased.
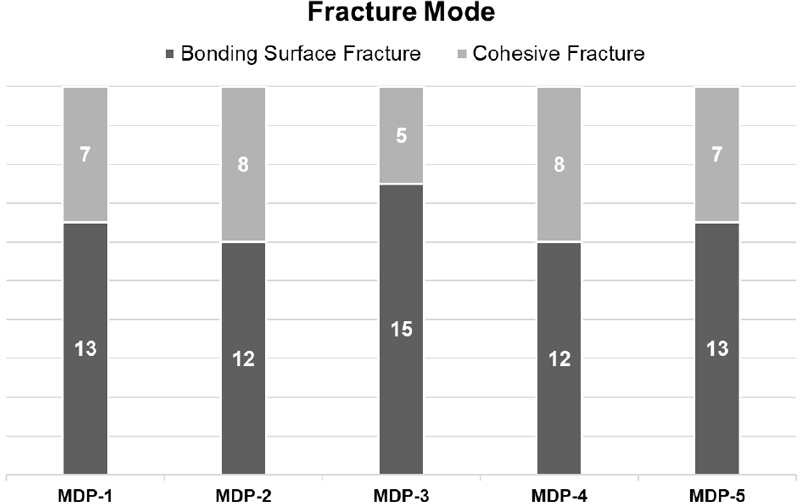
The results of fracture modes are also indicated in Table 3 as cohesive fractured specimens were marked. Additionally, the results are summarized in Figure 2. Observation of the de-bonded adhesive interface revealed that MDP-3, which had the highest bond strength, had the highest number of specimens exhibiting cohesive fracture with residual resin remaining on the tooth surface. This indicates a positive correlation between the number of specimens exhibiting cohesive fracture and the bond strength.
Different superscript lower case letters for average values indicate significant differences (p<0.05).
Discussion
This study examined immediate bonding strength depend on proportion of the 10-MDP in the experimental universal adhesive.10-MDP is well known as a bifunctional monomer, as with its ability to form nano layers to different material characteristics such as hydrophilic tooth structure and hydrophobic restorative material (9). The result of shear bond strength test demonstrated that increasing 10-MDP first increased the bonding strength. However, further increase in 10-MDP had limited effect on the bonding strength and resulted in decrease in value for MDP-4 and MDP-5g groups. This may be due to the fact that while an increase in the amount of functional monomer may be beneficial for the reaction with the hydroxyapatite of the tooth surface (9), its effect on the durability of the bonding layer would be limited as measurement is carried out for immediate bonding strength.
Increaseing proportion of 10-MDP result in lower pH of adhesives. This is presumed to occur because the hydrogen ions in MDP become activated upon contact with water and the solvent, ethanol, thereby lowering the pH of the experimental adhesives. During the process of adhesion, pH is an important factor in the adhesion compatibility between dental materials, including universal adhesives and aromatic tertiary amine-containing cements. The pH of a universal adhesive is typically acidic, which is lower than pH 3.0. Phosphate-containing monomers such as 10-MDP mixed with ethanol as a solvent result in activation of H+ ions in the monomer, leading to increase acidity in the mixture (10) . This low pH helps with the etching and bonding process by demineralizing the tooth surface and creating micromechanical retention. However, aromatic tertiary amine-containing cements cannot be cured at the interface to bonding layer when its pH is under 3.0. Universal adhesives are commonly used in restorative dentistry to bond composite materials to tooth structures. Aromatic tertiary amine-containing cements, on the other hand, are dental cements that contain chemicals such as benzoyl peroxide and tertiary amines, which are used for cementation purposes.
Universal adhesive refers to an adhesive that can be used with various materials, regardless of the etching method, type of restorative material including zirconia, or type of cement. It is designed to be versatile and compatible with a wide range of applications (11) . However, depends on the characteristics of substrates as a restorative and luting materials, there are some points to discuss. Universal adhesives containing 10-MDP, when diluted in a solvent, can maintain sufficient hydrophilicity to penetrate dentin tubules and form a hybrid layer. However, after application to the tooth and complete evaporation of the solvent followed by polymerization, the hydrophobic nature of 10-MDP, due to its high partition coefficient, allows for the implementation of a hydrophobic adhesive layer (12). In our experiment, we conducted contact angle tests on the experimental adhesive, but we didn't find any significant differences between the experimental groups. As the angles measured were consistently 30 degrees or higher, indicating hydrophobic characteristics, we concluded without further measurements (13).
Universal adhesive has limitations when it comes to bonding with ceramics without the addition of silane and compatibility with cements that contain benzoyl peroxide (BPO) and three-component amines in self-curing function due to its fundamental properties. Silane (3-Methacryloxyproyltrimethoxysilane) is activated through pure acidity and exposure to H2O. In its activated state, it renders the ceramic surface hydrophobic and prepares it for bonding with the adhesive resin. Silane is crucial for enabling chemical bonding with ceramics (14). However, universal adhesives rely on phosphate-based monomers that have been validated for bonding with zirconia. These monomers, upon contact with water-containing solvents, increase the concentration of hydrogen ions, resulting in acidification. Therefore, if silane is added to universal adhesive, it reacts prematurely in an already activated state before activating the ceramic surface (15). Research has shown that once silane is activated prematurely, it undergoes a self-curing reaction, transforming into silanol, and cannot interact with the ceramic surface. Pure silane has to use on ceramic surface for the bonding to the resin or sodium sulfinate accelerate generating free radical in acidic condition. Hence, the claim that a single bottle of universal adhesive can be used for all types of teeth and restorative materials is debatable, and improvements are needed to achieve such capabilities in the future.
The 10-MDP used in this study is a phosphate-based monomer. Previous research has shown that phosphate-based monomers exhibit higher bonding affinity to zirconia compared to carboxyl-based monomers. Zirconia surfaces do not etch with hydrofluoric acid, unlike traditional ceramics, and they are not affected by silane coupling agents. This is because zirconia surfaces lack silica. The possible reaction pathway of phosphate monomer with zirconia is, two hydrogen groups (from phosphoric acid group) will react slowly with one oxygen group (from zirconia), liberating a water molecule to from a stable Zr-O-P covalent bond (16). Based on this theoretical background, several previous studies have investigated adhesion to zirconia surfaces using phosphate monomers. This has been indirectly demonstrated through techniques such as contact angle measurements and SIMS (Secondary Ion Mass Spectrometer). Therefore, inferring from the use of phosphate monomers like 10-MDP as functional monomers, it can be deduced that universal adhesives can achieve bonding to zirconia surfaces as well (17).
10-MDP, which exhibits excellent bonding properties with zirconia and enamel, possesses an OH group at one end, which, upon encountering H2O, activates hydrogen ions, acidifying the entire adhesive solution. Previous studies have indicated that single-bottle, one-layer adhesives with a pH below 3.0 are incompatible with cements that have a reaction structure consisting of benzoyl peroxide (BPO) and aromatic tertiary amines (8). The theoretical background behind this research suggests that when the adhesive layer has a pH below 3.0, acidic monomers with a pH below 3.0 remaining in the oxygen-inhibited layer hinder the interaction between the amine component of the cement and BPO, preventing their bonding. Consequently, polymerization of the cement does not occur at the interface between the adhesive layer and the cement, preventing chemical bonding between the two layers (19). Considering two fact, such as mixing silane with 1 bottle universal adhesive and incompatibility with self-cure cement, it is evident that more research is required for single-bottle universal adhesives, to ensure compatibility with all restorative materials including ceramic and self-cure cements.
One of limitation of the study and related possible future study may be the measurement of hydrophilicity or surface energy. As stated earlier, our earlier results showed no significant differences among the experimental adhesive materials with contact angles. The results closely approximated those of commercially available products (30 degrees or more), indicating that the overall experimental materials met the necessary and sufficient conditions (20). Therefore, a comparative analysis for the contact angle study were not included in this study. Still, calculation of surface energy may be useful for hydrophobic materials in the future.
Conclusion
The results of this study concluded that as the amount of 10-MDP in the experimental dental universal adhesive increased, the pH decreased. In addition, when the experimental dental universal adhesive was applied to the tooth specimen and resin was bonded, the shear bond strength increased gradually with increasing amounts of 10-MDP, but showed a tendency to decrease gradually after a certain amount was exceeded.
Through this study, the universal adhesive used in the experiment showed the highest adhesive strength at 9.710 wt% of 10-MDP content, with a pH of 2.86 for that experimental adhesive group. Based on the results of this study, this composition of 10-MDP can be considered the most ideal for clinical application, though additional experiments would be warranted as the future studies such as biological evaluation and clinical studies.
References
-
Jang JH, Lee MG, Woo SU, Lee CO, Yi JK, Kim DS. Comparative study of the dentin bond strength of a new universal adhesive. Dent Mater J. 2016;35(4):606-12.
[https://doi.org/10.4012/dmj.2015-422]

-
Carrilho E, Cardoso M, Marques Ferreira M, Marto CM, Paula A, Coelho AS. 10-MDP based dental adhesives: adhesive interface characterization and adhesive stability—a systematic review. Materials (Basel). 2019;12(5): 790.
[https://doi.org/10.3390/ma12050790]

-
Valente F, Mavriqi L, Traini T. Effects of 10-MDP Based Primer on Shear Bond Strength between Zirconia and New Experimental Resin Cement. Materials (Basel). 2020;13(1):235.
[https://doi.org/10.3390/ma13010235]

-
Yoshida Y, Yoshihara K, Hayakawa S, Nagaoka N, Okihara T, Matsumoto T, et al. HEMA inhibits interfacial nano-layering of the functional monomer MDP. J Dent Res. 2012;91(11):1060-5.
[https://doi.org/10.1177/0022034512460396]

-
Luque-Martinez IV, Perdigão J, Muñoz MA, Sezinando A, Reis A, Loguercio AD. Effects of solvent evaporation time on immediate adhesive properties of universal adhesives to dentin. Dent Mater. 2014;30(10):1126-35.
[https://doi.org/10.1016/j.dental.2014.07.002]

-
Yoshida Y, Van Meerbeek B, Nakayama Y, Yoshioka M, Snauwaert J, Abe Y, et al. Adhesion to and decalcification of hydroxyapatite by carboxylic acids. J Dent Res. 2001;80(6):1565-9.
[https://doi.org/10.1177/00220345010800061701]

- O'Keefe KL, Powers JM. Adhesion of resin composite core materials to dentin. Int J Prosthodont. 2001;14(5):451-6.
-
Ekambaram M, Yiu CKY, Matinlinna JP. An overview of solvents in resin–dentin bonding. Int J Adhes Adhes 2015;57:22-33.
[https://doi.org/10.1016/j.ijadhadh.2014.09.007]

-
Dabsie F, Grégoire G, Sharrock P. Critical surface energy of composite cement containing MDP (10-methacryloyloxydecyl dihydrogen phosphate) and chemical bonding to hydroxyapatite. J Biomater Sci Polym Ed. 2012;23(1-4):543-54.
[https://doi.org/10.1163/092050611X554480]

- Chen L, Suh BI. Effect of hydrophilicity on the compatibility between a dual-curing resin cement and one-bottle simplified adhesives. J Adhes Dent. 2013;15(4):325-31.
-
Nagaoka N, Yoshihara K, Feitosa VP, Tamada Y, Irie M, Yoshida Y, et al. Chemical interaction mechanism of 10-MDP with zirconia. Sci Rep. 2017;7:45563.
[https://doi.org/10.1038/srep45563]

-
Feitosa VP, Ogliari FA, Van Meerbeek B, Watson TF, Yoshihara K, Ogliari AO, et al. Can the hydrophilicity of functional monomers affect chemical interaction? J Dent Res. 2014;93(2):201-6.
[https://doi.org/10.1177/0022034513514587]

-
Feitosa VP, Sauro S, Ogliari FA, Ogliari AO, Yoshihara K, Zanchi CH, et al. Impact of hydrophilicity and length of spacer chains on the bonding of functional monomers. Dent Mater. 2014;30(12):e317-23.
[https://doi.org/10.1016/j.dental.2014.06.006]

-
Kim YR, Kim JH, Son SA, Park JK. Effect of Silane-Containing Universal Adhesives on the Bonding Strength of Lithium Disilicate. Materials (Basel). 2021;14(14):3976.
[https://doi.org/10.3390/ma14143976]

-
Yao C, Yu J, Wang Y, Tang C, Huang C. Acidic pH weakens the bonding effectiveness of silane contained in universal adhesives. Dent Mater. 2018;34(5):809-18.
[https://doi.org/10.1016/j.dental.2018.02.004]

- Suh BI. Principles of adhesion dentistry: a theoretical and clinical guide for dentists. AEGIS Communications. 1st ed. Chicago: Agis Communication; 2013.
-
Llerena-Icochea AE, Costa RM, Borges A, Bombonatti J, Furuse AY. Bonding Polycrystalline Zirconia With 10-MDP-containing Adhesives. Oper Dent. 2017;42(3):335-41.
[https://doi.org/10.2341/16-156-L]

-
Bolhuis PB, de Gee AJ, Kleverlaan CJ, El Zohairy AA, Feilzer AJ. Contraction stress and bond strength to dentinfor compatible and incompatible combinations of bonding systems and chemical and light-cured core build-up resin composites. Dent Mater. 2006 Mar;22(3):223-33.
[https://doi.org/10.1016/j.dental.2005.03.016]

-
Franco EB, Lopes LG, D'Alpino PH, Pereira JC. Influence of pH of different adhesive systems on the polymerization of a chemically cured composite resin. Braz Dent J. 2005;16(2):107-11.
[https://doi.org/10.1590/S0103-64402005000200004]

-
Balkaya H, Demirbuğa S. Evaluation of six different one-step universal adhesive systems in terms of dentin bond strength, adhesive interface characterization, surface tension, contact angle, degree of conversion and solvent evaporation after immediate and delayed use. J Esthet Restor Dent. 2023;35(3):479-92.
[https://doi.org/10.1111/jerd.12973]