
Relationship of tooth loss and Implant related to periodontal treatment among periodontal disease patients with hypertension
Abstract
본 연구에서는 치주염 환자에서 치아상실과 고혈압, 임플란트 치료와 고혈압 사이의 상관관계를 알아보기 위해 조사한 것으로 중등도에서 중증까지의 치주염을 가진 고혈압 환자군(n=267)과 대조군(n=291)에서 초진 시 치아상실 및 임플란트 치료 수, 치주치료 중 치아상실의 수를 평가하였다. 수집된 자료의 통계처리는 SPSS 프로그램으로 비교하였다.
결과적으로 초진 시 치아 상실 수는 두 군에서 분명한 차이를 보였다(OR:2.05). 비록 통계적으로 유의성은 없었지만 임플란트 치료 수는 고혈압 환자군에서 더 높게 나타났다. 고혈압은 진전된 치주질환과 연관이 있으며, 이로 인한 발치 시 임플란트 처치는 조절성 고혈압 환자에 있어서 고려할 만한 수복치료 방법으로 생각된다.
결론적으로 고혈압과 치아상실 사이에는 유의한 연관성이 관찰되었으며, 고혈압 환자에 서 고혈압 치료 및 관리와 함께 유지 치주 치료 및 구강위생 교육이 필요할 것으로 생각된다.
Keywords:
Chronic periodontitis, HypertensionⅠ. INTRODUCTION
In general, cardiovascular diseases appear to have a number of characteristics. They are more likely to occur in older people, males, smokers, hypertensive patients, people with lower educational status, those with fewer financial resources, and socially isolated and stressed people (Beck JD et al., 2000).
Cardiovascular disease includes several diseases: artherosclerotic cardiovascular disease, hemorrhagic stroke, congestive heart failure, hypertension, rheumatic heart disease and congenital heart defects.
Hypertension is very common disease in Korea. On average 28% of Koreans reported that they had hypertension in 2007. Moreover, prevalence of hypertension is increasing (Hosik Min et al., 2010). .
Hypertension is related to inflammation (Boos CJ & Lip GY, 2005). Elevated inflammatory markers, such as interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α) were associated with increased risk of hypertension (Sesso HD et al., 2003) and were fairly related to elevated blood pressures.
Patients with moderate to severe alveolar bone resorption have an increased prevalence of systemic diseases, especially hypertension and diabetes mellitus (Al-Emadi et al., 2006).
Periodontal disease has a number of common risk factors with cardiovascular disease, such as age, male, socio-educational status, and smoking. Accordingly the question arises as what the nature of association between periodontitis and cardiovascular disease.
Risk factors for tooth loss include dental caries (Phipps KR & Stevens VJ, 1995), periodontal disease (Phipps KR & Stevens VJ, 1995; Beck JD et al., 1997), smoking habit (Krall EA et al., 1999), lower socioeconomic status and irregular dental visits (Gilbert GH et al., 1999), and osteoporosis (Krall EA et al., 1999; Mohammad AR et al., 1997). Dental caries is a major reason for tooth loss in all age-groups, on the other hand periodontal disease is the most frequent reason of tooth extraction for people over 40 years of age (Reich E & Hiller KA, 1993).
Periodontal disease is characterized by a chronic infection and inflammation in the periodontal tissue leading to the destruction of the alveolar bone and, finally, to tooth loss (Page RC et al., 2000). Periodontal disease is associated with raised systemic concentration of IL-6, CRP, fibrinogen, and TNF-α (Page RC et al., 2000), and periodontal treatment may decrease blood levels of these inflammatory markers. Moreover, it is suggested that CRP and M-CSF might be inflammatory and bone resorption markers in periodontal disease compromised tissue. It is assumed that hypertension may be associated with the progression of periodontal inflammation and alveolar bone resorption (Chul-Woo Kim et al., 2009).
The presence of periodontal pathogens-including Prevotella intermedia, Porphyromonas gingivalis, and Tanerella forsynthesis from subgingival plaque samples was elevated CRP levels (P=0.029). CRP levels were also reported to be higher in 50 cardiovascular disease patients with severe periodontitis (>4mm deep gingival pockets) than in 46 healthy cases. It was reported that both CRP and serum fibrinogen concentrations were significantly higher in cardiovascular disease patients than in controls (Meurman JH et al., 2003).
Chronic periodontal disease is associated with an increased risk of cardiovascular complications (Blaizot A et al., 2009). One of the possible explanations for this is that bacteria and bacterial toxins present in dental plaque and crevicular fluid stimulate immune cells to release a number of inflammatory mediators (Bodet C et al., 2006), which act locally but also pass into the systemic circulation.
In animal experiments, Streptococcus sanguis, a predominant oral microorganism, induces platelet aggregation, an important thrombotic process in arterial plaque formation (Herzberg MC et al., 1992). Destructive periodontal disease, which involves Gram-negative bacteria, has been reported to be a significant predictor of coronary heart disease (Beck J et al.,1996). Because both coronary heart disease and periodontal disease have a multifactorial etiology, as well as a wide variety of possible confounding factors, a clear cut consensus on the importance of the relationship between these two conditions has been difficult to obtain.
Local chronic bacterial infection periodontal tissue may influence systemic levels of inflammatory mediators and this may contribute to endothelial dysfunction, carotid artery plaque formation or deterioration of the anti-atherogenic potency of high-density lipoproteins (Desnarieux et al., 2003; Pussinen et al., 2004).
A relationship between periodontal disease and cardiovascular disease has been reported in many studies. In spite of increasing evidence of animal and population based studies that show an association between periodontal disease and cardiovascular disease further research is needed to determine the extent of these associations. The relation between tooth loss and cardiovascular diseases is important owing to the high prevalence of periodontal disease and tooth loss.
The relationship between hypertension and periodontal disease was previously evaluated in earlier studies. With the emergence of periodontal medicine, most studies focused on periodontal infection as a source of pathogenic species and inflammatory mediators that can create a systemic inflammatory burden and increase the risk for hypertension and the development of other cardiovascular disorders. However, some studies focused on the impact of the hypertensive status on periodontal tissues, and even fewer reports evaluated the effects of elevated blood pressure on tooth-supporting alveolar bone in the presence of an infectious challenge and under healthy local conditions.
The purpose of the present study is to assess the existence of association of tooth loss and hypertension, implant treatment and hypertension among periodontal disease patients.
Ⅱ. MATERIALS AND METHODS
Two groups of patients with hypertension and controls were investigated. The patients in the both groups comprise moderate and severe chronic periodontitis (1999, American Academy of Periodontology).
Hypertension was assessed as self-reported physician diagnosis as well as treatment of hypertension drug. Self-reported history of hypertension collected in 267 patients referred to the Department of Periodontology, Kyungpook National University Dental Hospital, for periodontal treatment between years 2009 and 2010 and 291 subjects randomly selected from patients without medical history. The study protocol was reviewed and accepted by Research Ethics Committee, Kyungpook National University (Ethics Reference No. KNUH 2012-06-023-001).
All patients asked their age, gender, past medical history, smoking habit. The tooth loss and history of implant or crown and bridge treatment was assessed by recording of initial oral examination and radiographic analysis. The loss of teeth over the treatment period was determined by comparing the initial and re-examination charting, radiographic finding and reviewing the treatment history in the chart.
All patients were treated non-surgical and/or surgical cause-related periodontal therapy consisting of oral hygiene instructions, extraction of hopeless teeth, dental implantation performed by periodontists.
1. Data analysis
Continuous data were presented as mean and standard deviation. Nominal data were expressed as absolute numbers and percent value. Multivariable modeling was performed using binary logistic regression. The authors examined the distribution of age, gender and smoking across tooth loss categories. The unadjusted prevalence of tooth loss, history of implant treatment, crown and bridge treatment at first visit, tooth loss during treatment period and odds ratio (ORs) were calculated for the association between hypertension and tooth loss, history of implant, crown and bridge treatment at first visit and tooth loss during treatment using logistic regression.
Multivariate analysis was performed to examine which factors found significant with unvariate analyses remained as such after adjusting for confounding factors. Age was categorized into four categories as follow: 40-49, 50-59,60-69, and ≥70years old. In all analyses, age category of 40-49 was the reference group. Variables entered in the model were gender (male or female), smoking status (smoker versus non-smoker).
To detect possible interactions, Tooth loss at first years was categorized as two groups (tooth loss <5, tooth loss ≥5). History of implant and crown and bridge treatment at first visit and tooth loss during treatment divided into two groups (none and one or more).
Statistical analyses and data management were performed using statistical software program (Statistical Package for the Social Sciences v.21.0). Adjusted odds ratios (OR) and corresponding 95% confidence intervals were generated for all significant variables. The significance level used was P<0.05.
Ⅲ. RESULTS
A total of 267 people aged 40-80 years of hypertension group and 291 people aged 40-80 years of control group were considered for analysis. Basic characteristics of the Study population are given in Table 1.
As seen in Figure 1, there was a significant difference between tooth loss at first visit in hypertension group and that in control group.
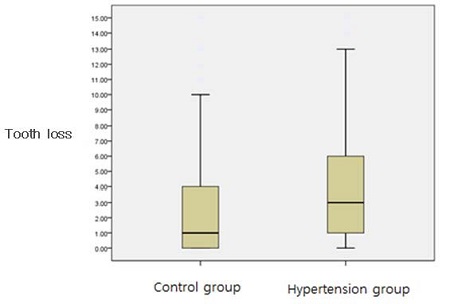
Box plot of number of tooth loss at first visit. Hypertension group show significantly higher number of tooth loss at first visit compared to controls.
1. Hypertension and tooth loss at first visit
There was a significant association between tooth loss and hypertension (OR 2.92), age and smoking. After adjustment for hypertension, age and smoking, there were significant difference in the number of tooth loss at first visit between hypertension and controls. Adjusting for age and smoking, patients with hypertension had a 2.05 relative risk compared to patients without hypertension. Hypertension is associated with an increased risk for periodontal disease, particularly in patients over the age of 50(Table 2).
2. Hypertension and the history of implant treatment at first visit
Considering interactions between history of implant treatment at first visit with hypertension, age, gender, smoking status, none of them revealed statistical significance (Table 3). However, hypertensive patients were treated more number of implant restoration than controls.
3. Hypertension and the history of crown & bridge treatment at first visit
Subjects with Hypertension had a significantly higher bridge treatment history compared with control subjects after adjusting for confounders (OR: 2.132, 95% confidence interval[CI]: 1.468 to 3.095) (Table 4).
Ⅳ. DISCUSSION
Hypertension was reported to be associated with progression of periodontal disease. A number of studies have reported periodontal disease is associated with hypertension and cardiovascular disease (Beck J et al., 1996; Destefano F et al., 1993; Mattila K et al., 1995; Morrison HI et al., 1999), while other studies have not (Howell TH et al., 2001; Huojel PP et al., 2000; Joshipura K et al., 2002; Mattila KJ et al., 2000). This study strongly suggests that periodontal condition is associated with hypertension. Tooth loss can be an outcome of severe periodontal disease. In the present study, a strong association between hypertension and the number of tooth loss before periodontal treatment after adjusting for confounders.
Implant treatment history of hypertensive patients was higher odds ratio, although being not significantly different in this study. It can be suggested that tooth loss was positively associated with hypertension and hypertension is not a risk factor in implant treatment comparing to healthy people. In retrospective cohort study suggested that hypertension is no absolute contraindications in implant placement.
It is interesting that the association with crown and bridge treatment is expressed by higher odds ratios than the association with implant treatment. It is suggested that hypertension patients who had visited department of periodontics in Kyungpook national university hospital preferred crown and bridge treatment to implant restoration.
Tooth loss is significantly associated with hypertension after controlling for confounding factors.
However, active treatment of hypertension patient could reduce the number of tooth loss and improve the periodontal inflammation. Non-surgical and surgical periodontal therapy was effective in improving periodontal status in hypertensive patients with moderate to severe periodontitis.
This study has some limitations. One of the limitations of this study was the inability to assess cause of tooth loss exactly. This study was unable to examine whether the periodontal disease was a cause of the tooth extraction. However, the majority of tooth extraction in patients older than 35 years of teeth is lost for periodontal reasons (Khalaf F et al., 2005). In the same age range of the subjects, this study is hypothesized that the tooth loss caused by periodontal disease, hypertension group was significantly high number of tooth loss at first visit. It means that hypertension is associated with the progression of periodontal disease.
Another possible limitation of this study is onset of the hypertension. There is a limit to compare two groups because hypertension patients did not suffer same period. Moreover, because the hypertension history is based on self-reported data that have not validated, these results could be influenced by reporting biases.
Diet and chronic inflammatory mediators have been considered as two major casual pathways linking periodontal disease and cardiovascular disease.
In a report based on the National Health and Nutrition Epidemiologic follow-up study (NHEFS), a 14-year follow-up of 9760 participants, Destefano et al. (1993) reported relative risks (RR) for myocardial infarction of 1.23 (95%CI, 1.05 to 1.44) among people with no gingivitis or periodontitis. The risk associated with edentulousness was similar to that for periodontal disease in this population
Several possible pathways for the relationship between periodontal disease and CVD have been postulated. Periodontal disease may increase systemic levels of inflammatory mediators and thus potentially contribute to the inflammation-associated atherosclerotic process (Meurman JH et al., 2004). Periodontal pathogens may also disseminate into the systemic circulation and localize in atheromas (Chun YH et al., 2005). Alternatively, individuals with periodontal disease and CVD may share common behaviors or have common host responses to inflammation. The results of periodontal disease and tooth loss may lead to dietary changes, such as decreased intake of fruits and vegetables/dietary fiber, that could subsequently affect the risk for CVD and other disease. Also, those who genetically susceptible to systemic inflammation may demonstrate increased oral inflammation in forms of gingivitis or periodontal disease as well as increased risk of CVD. Because of this complexity, it is difficult to assess whether oral disease actually contributes to increased risk of CVD or whether oral disease and CVD share common risk factors.
A relationship between tooth loss and hypertension is of considerable interest because of the prevalence of both conditions in the general population.
In conclusion, a significant association was observed between the extent of tooth loss and hypertension. Therefore, prevention and control of hypertension, supportive periodontal therapy and oral hygiene education should be recommended for hypertension patients.
References
- Al-Emadi, A., Bissada, N., Farah, C., Siegel, B., Al-Zaharani, M., (2006), Systemic diseases among patients with and without alveolar bone loss, Quintessence Int, 37, p761-765.
-
Beck, J., Garcia, R., Heiss, G., Vokonas, PS., Offenbacher, S., (1996), Periodontal disease and cardiovascular disease, J periodontal, 67, p1123-1137.
[https://doi.org/10.1902/jop.1996.67.10s.1123]

-
Beck, JD., Sharp, T., Koch, GG., Offenbacher, S., (1997), A 5-year study of attachment loss and tooth loss in community dwelling older adults, J Periodontal Res, 32, p516-523.
[https://doi.org/10.1111/j.1600-0765.1997.tb00567.x]

-
Beck, JD., Slade, G., Offenbacher, S., (2000), Oral disease, cardiovascular disease and systemic inflammation, Periodontol 2000, 23, p110-120.
[https://doi.org/10.1034/j.1600-0757.2000.2230111.x]

- Blaizot, A., Vergnes, J N., Nuwwareh, S., Amar, J., Sixou, M., (2009), Periodontal diseases and cardiovascular events: a meta-analysis of observational studies, International Journal of Dentistry, 59, p197-209.
-
Bodet, C., Chandad, F., Grenier, D., (2006), Porphyromanas gingivalis-induced inflammatory mediator profile in an ex vivo human whole blood model, Clinical and Experimental Immunology, 143, p50-57.
[https://doi.org/10.1111/j.1365-2249.2005.02956.x]

-
Boos, CJ., Lip, GY., (2005), Elevated high-sensitive C-reactive protein, large arterial stiffness and atherosclerosis: a relationship between inflammation and hypertension?, J Hum Hypertens, 19, p511-513.
[https://doi.org/10.1038/sj.jhh.1001858]

- Chu, -Woo Kim, Jin-Woo Park, Jo-Young Suh, Je-Yoel Cho, Jae-Mok Lee., (2009), The expressions of C-reactive protein and macrophage colony-stimulating factor in gingival tissue of human chronic periodontitis with hypertension, J Korean Acad Periodontol, 39, p391-398.
-
Chun, YH., Chun, Kr, Olguin D., et al , (2005), Biological foundation for periodontitis as a potential risk factor for atherosclerosis, J Periodontal Res, 40, p87-95.
[https://doi.org/10.1111/j.1600-0765.2004.00771.x]

-
Destefano, F., Anda, R., Kahn, H., et al , (1993), Dental disease and risk of coronary heart disease and mortality, Br Med J, 306, p688-69.
[https://doi.org/10.1136/bmj.306.6879.688]

-
Gilbert, GH., Miller, MK., Duncan, RP., Ringelberg, ML., Dolan, TA., Foerster, U., (1999), Tooth-specific and person-level predictors of 24 month tooth loss among older adults, Community Dent Oral Epidemiol, 27, p372-385.
[https://doi.org/10.1111/j.1600-0528.1999.tb02034.x]

- Herzberg, MC., MacFarlane, GD., Gong, K., Armstrong, NN., Witt, AR., Erickson, PR., et al , (1992), The platelet interactivity phenotype of Streptococcus sanguis influences the course of experimental endocarditis, Infect Immun, 60, p4809-4818.
-
Hosik Min, JongWha Chang, Rajesh Balkrishnan, (2010), Sociodemographic Risk Factors of Diabetes and Hypertension Prevalence in Republic of Korea, International Journal of Hypertension, p1-6.
[https://doi.org/10.4061/2010/410794]

-
Howell, TH., Ridker, PM., Ajani, UA., Hennekens, CH., et al , (2001), Periodontal disease and risk of subsequent cardiovascular disease in U.S. male physicians, J Am Coll Cardiol, 37, p445-450.
[https://doi.org/10.1016/S0735-1097(00)01130-X]

-
Huojel, PP., Drangsholt, M., Spekerman, C., DeRouen, TA., (2000), Periodontal disease and coronary heart disease risk, JAMA, 284, p1406-1410.
[https://doi.org/10.1001/jama.284.11.1406]

- Joshipura, K., Rimm, E., Douglass, C., Trichpoulos, D., et al , (2002), Poor oral health and coronary heart disease, J Dent Res, 81, p186-191.
-
Khalaf F. Al-Shammari, Areej K. Al-Khabbaz, Jassem M. Al-Ansari, (2005), Rodrigo Neiva, and Hom-Lay Wang Risk indicators to tooth loss due to periodontal disease, J Periodontol, 76, p1910-1918.
[https://doi.org/10.1902/jop.2005.76.11.1910]

-
Krall, EA., Garvey, AJ., Garcia, RI., (1999), Alveolar bone loss and tooth loss in male cigar and pipe smokers, J Am Dent Assoc, 130, p57-64.
[https://doi.org/10.14219/jada.archive.1999.0029]

-
Mattila, K., Valtonen, V., Nieminen, M., Huttunen, J., (1995), Dental infection and the risk of new coronary events: Prospective study of patients with documented coronary artery disease, Clin Infect Dis, 20, p588-592.
[https://doi.org/10.1093/clinids/20.3.588]

-
Mattila, KJ., Asikainen, S., Wolf, J., et al , (2000), Age, dental infections, and coronary heart disease, J Dent Res, 79, p756-760.
[https://doi.org/10.1177/00220345000790020901]

-
Meurman, JH., Janket, S-J., Qvarnström, M., Nuutinen, P., (2003), Dental infections and serum inflammatory markers in patients with and without severe heart disease, Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 96, p695-700.
[https://doi.org/10.1016/j.tripleo.2003.08.017]

-
Meurman, JH., Sanz, M., Janket, SJ., (2004), Oral health, atherosclerosis, and cardiovascular disease, Crit Rev Biol Med, 15, p403-413.
[https://doi.org/10.1177/154411130401500606]

- Mohammad, AR., Bauer, RL., Yeh, CK., (1997), Spinal bone density and tooth loss in a cohort of postmenopausal women, Int J Prosthodont, 10, p381-385.
- Morrison, HI., Ellison, LF., Taylor, GW., (1999), Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases, J Cardiovasc Risk, 6, p7-11.
-
Page, RC., Offenbacher, S., Schroeder, HE., Seymour, GJ., Kornman, KS., (1997), Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions, Periodontol 2000, 14, p216-248.
[https://doi.org/10.1111/j.1600-0757.1997.tb00199.x]

-
Phipps, KR., Stevens, VJ., (1995), Relative contribution of caries and periodontal disease in adult tooth loss for an HMO dental population, J Public Health Dent, 55, p250-252.
[https://doi.org/10.1111/j.1752-7325.1995.tb02377.x]

-
Reich, E., Hiller, KA., (1993), Reasons for tooth extraction in the western states of Germany, Community Dent Oral Epidemiol, 21, p379-383.
[https://doi.org/10.1111/j.1600-0528.1993.tb01103.x]

-
Sesso, HD., Burning, JE., Rifai, N., Blake, GJ., Gaziano, M., Ridker, PM., (2003), C-reactive protein and the risk of developing hypertension, JAMA, 290(22), p2945-51.
[https://doi.org/10.1001/jama.290.22.2945]


