
Influence of Adhesive Layer Thickness of Dentin Adhesives on Microtensile Bond Strength of a Resin Composite to Dentin
초록
상아질 접착제의 올바른 선택과 사용은 성인 뿐 아니라 소아 환자에서 신속하고 정확한 레진 수복에 있어서 매우 중요하다. 본 연구에서는상아질 접착제의 본딩 레진의 두께가 복합 레진의 상아질에 대한 미세인장 접착강도에 미치는 영향을 평가하였다. 상아질 접착제로는 All-Bond 2와 All-Bond 3를 사용하였다. 본딩 레진의 두께를 50, 100, 200 µm으로 조절하기 위해서, 해당 두께를 가진 접착성 테이프를 연마된 상아질 면에 접착시켰고, 그 외의 접착 단계는 제조자의 지시를 준수하였다. 접착된 시편은 24시간 37℃ 증류수에 보관하였고, 다이아몬드 쏘를 이용하여 미세인장 접착용 시편들을 제작한 다음 (접착면적 : 0.8 mm2 (±0.1)) 만능시험기를 이용하여 0.5 mm/min의속도로 시편을 파절시켜 미세인장 접착강도를 MPa 단위로 구하였다. 실험 결과 All-Bond 2에서는 중간 두께인 100 µm에서 가장 높은접착강도를 나타내었고, 그 다음 200 µm이었으며, 50 µm에서 가장 낮은 접착강도를 보였다. 한편 All-Bond 3에서는 접착강도가 본딩레진의 두께에 영향을 받지 않았다. 이는 주로 All-Bond 2의 본딩 레진의 주성분인 2-hydroxyethyl methacrylate(HEMA)의 친수성/소수성과관련된 것으로 생각된다. 본딩 레진의 두께가 복합 레진의 상아질 접착에 미치는 영향은 접착제에 따라 다르게 나타날 수 있으므로 주의가필요하다.
키워드:
상아질 접착제, 두께, 미세인장 접착강도, 복합 레진Ⅰ. INTRODUCTION
While the importance of hybrid layer is emphasized through many studies, less studies are done about the adhesive layer; that is, the hydrophobic layer overlying the hybrid layer (Kim et al. 2014). As the component overlying the hybrid layer, the adhesive layer may help to protect the bonding interface from polymerization shrinkage stresses and act as a stress absorbing layer (Cadenaro et al. 2008; Kwon et al. 2012).
A number of studies have evaluated the role of the elasticity of the hybrid layer and/or adhesive resin layer in relieving the polymerization stress of resin composites. Although there seems to be no relationship between the thickness of the hybrid layer and tensile bond strength, concern remains that thin hybrid layers may not provide as much stress-breaking function as thicker hybrid layers (El Zohairy et al. 2005). However, one possible solution is to use thicker adhesive layers on top of thin hybrid layers.
Since there is limited information in the dental literature correlating bond strengths and thickness of the adhesive resin layer, this study evaluated the relationship between the thickness of the adhesive resin layer and microtensile bond strength. The hypothesis is that increased thickness of the adhesive layer would result in higher bond strengths by improving stress distributions in the bonding interface.
Ⅱ. MATERIALS AND METHODS
Two commercially available dentin adhesives (All-Bond 2 and All-Bond 3, both from Bisco, Inc.) were used. Tetric Ceram (Ivoclar Vivadent) was used as the composite resin. Their manufacturers, lot numbers are summarized in Table 1.
Non-carious human molars were collected after obtaining the patients’ informed consents obtained under a protocol approved by the Ethics Committee of the School of Dentistry, Kyungpook National University. The teeth were stored in 0.5% chloramine in water at 4℃ and used within one month following extraction. The occlusal enamel and roots of the teeth were removed using a slow-speed saw with a diamond-impregnated disk (Isomet, Buehler Ltd., Lake Bluff, IL, USA) under water cooling to form 5-6 mm thick, parallel-sided crown segments (Tay et al. 2003). A 600-grit silicon carbide paper was used under running water to create a thin smear layer on the dentin surface (Mazzitelli et al. 2008).
An adhesive tape with a 10 mm diameter hole was placed on the center of the dentin surface to limit the bonding surface area. The three different thickness (50, 100, and 200 μm) of the adhesive layers were controlled by the thickness of the adhesive tape (Table 2). The teeth samples were then randomly divided into six experimental groups (Table 2). The two dentin adhesives used according to the manufacturer’s instructions (Table 3) except for the control of the adhesive layer thickness. After applying the bonding resins, the dentin surface was then built up with a resin composite (Tetric Cram, Ivoclar Vivadent) to a height of 5 mm on three increments, light curing each increment for 20 s. All the bonded samples were stored in water at 37℃ for 24 h (Kim et al. 2014).
Each bonded specimen was longitudinally sectioned into 0.9 mm-thick slabs with the slow-speed diamond saw (Isomet). Each slab was fixed on a glass platform with sticky wax and serially sectioned into 0.8 mm2 (±0.1) sticks, in accordance with the “non-trimming” method of the microtensile test (Mazzitelli et al. 2008; Di Hipólito et al. 2012). The exact dimensions of each stick were measured using a digital caliper to calculate the precise cross-sectional area. For microtensile bond strength testing, three sticks were sectioned from the center of each bonded specimen (Hiraishi et al. 2009). The microtensile bond strength of each bonded specimen was recorded as the average of the three readings (Kim et al. 2015). Sticks with premature bond failure were assigned a null bond strength value and were included in the compilation of the mean bond strength as well as the failure mode assessment (Yip et al. 2001). The bonded composite-dentin sticks were attached to a testing device with cyanoacrylate glue (Zapit, DVA, Corona, CA, USA). The device was attached to a microtensile bond tester (Bisco Inc.) and loaded in tension at a crosshead speed of 0.5 mm/min until failure (Yip et al. 2001).
After fracturing, all specimens were examined under a stereomicroscope (Olympus, Tokyo, Japan) at a magnification of 20× and failure modes were categorized as: (a) adhesive failure along the dentin-resin interface; (b) cohesive failure of the dentin (Cohesive D), and (c) cohesive failure of the resin (Cohesive R) (El Zohairy et al. 2005).
The cross-sectional view of the bonding interface was also examined under a field emission-scanning electron microscope (FE-SEM, JSM-6700F, Jeol, Tokyo, Japan). The specimens were dehydrated through a series of ascending ethanol concentrations (70%, 80%, 95%, three changes in 100%) for 2 hours each, and then left to completely dehydrate in absolute ethanol for an additional 48 hours (Tay et al. 2003). Prior to SEM analysis, the specimens were finally air-dried and sputter-coated with platinum (Hiraishi et al. 2009).
All the data were statistically analyzed by parametric methods at α = 0.05 because they did not meet the homogeneity of variances assumption (Levene’s test). The Kruskal-Wallis test was used to compare the groups, followed by the Mann-Whitney post hoc test, with adjustment of significance levels using the Sidak correction for multiple testing (Yan et al. 2010). All the statistical analyses were performed using SPSS 17.0 for Windows (SPSS Inc, Chicago, IL, USA).
Ⅲ. RESULTS AND DISCUSSION
Figures 1 and 2 show the cross-sectional SEM images of the All-Bond 2 and All-Bond 3-applied groups. According to the photos, the adhesive layers with different thickness, along with the hybrid layer, were well-developed. The formation of the resin tags was also observed.
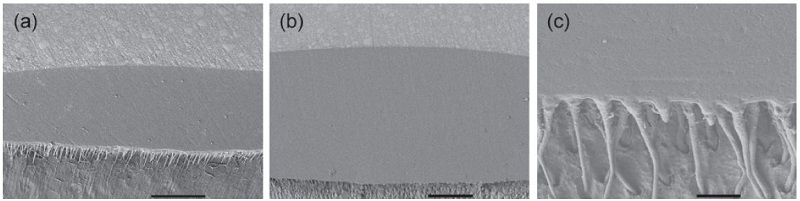
Cross-sectional SEM images of the All-Bond 2-applied groups: (a) 50 μm (250×, bar = 100 μm); (b) 100 μm (200×, bar = 100 μm); and (c) 200 μm (2,000×, bar = 10 μm).
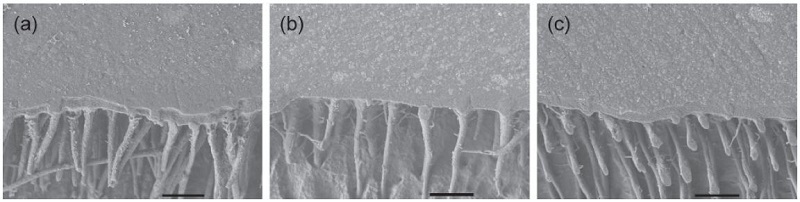
Cross-sectional SEM images of the All-Bond 3-applied groups (2,000×, bar = 10 μm): (a) 50 μm; (b) 100 μm; and (c) 200 μm.
Table 4 shows the microtensile bond strength values, along with the failure pattern analysis results. The influence of adhesive layer thickness on microtensile bond strength to dentin was found to be material-specific. In the case of All-Bond 2, the relatively thick (100 or 200 μm) adhesive layer produced a superior resin bonding to dentin compared with the thin (50 μm) adhesive layer. In contrast, as regard to All-Bond 3, the thickness of the adhesive layer did not significantly affect the microtensile bond strength. These findings may be due to the property or quality of the adhesive layer, rather than that of hybrid layer.
Recent advances in adhesive dentistry have seen a reduction in the application steps of enamel/dentin adhesives to meet the clinical demand for simplified, less technique sensitive bonding procedures (Suh et al. 2003; Kwon et al. 2012). However, the changes in formulation to develop simplified adhesives entails more acidic, and as a result, more hydrophilic adhesives (Tay & Pashley 2003). As the bonding resin of three-step etch and rinse, and two-step self-etch adhesives, do not contain either acidic monomers or solvents, they form a neutral, relatively hydrophobic adhesive layer prior to the placement of the filling (Kwon et al. 2012). On the contrary, two-step etch and rinse, or one-step self-etch adhesives (simplified adhesives), tend to retain a thin, uncured (i.e. oxygen inhibited) acidic, hydrophilic adhesive layer (Rueggeberg & Margeson 1990; Suh 2004).
In this present study, two three-step total-etch dentin adhesives were tested (Table 1). Thus, the adhesive layers for both dentin adhesives were basically hydrophobic. However, All-Bond 2 bonding resin (D/E Resin) contain 30% 2-hydroxyethyl methacrylate (HEMA), which is a small monomer that is in widespread use, not only in dentistry (Van Landuyt et al. 2007). Its popularity in medical applications must be attributed to its relatively good biocompatibility, even though the uncured monomer is notorious for its high allergenic potential. Uncured HEMA presents as a fluid that is well solvable in water, ethanol and/or acetone. Moreover, HEMA has been described to be able to evaporate from the adhesive solutions, though only in very small amounts (Pashley et al. 1998). Another important characteristic of HEMA is its hydrophilicity. Even though this monomer cannot be used as a demineralizing agent, its hydrophilicity makes it an excellent adhesionpromoting monomer. By enhancing wetting of dentin, HEMA significantly improves bond strengths. Nevertheless, both in uncured and cured state, HEMA will readily absorb water. HEMA in the uncured adhesive may absorb water, which can lead to dilution of the monomers to the extent that polymerization is inhibited (Van Landuyt et al. 2007). HEMA fixed in a polymer chain after polymerizing will still exhibit hydrophilic properties and will lead to water uptake with consequent swelling and discoloration (Burrow et al. 1999). Apart from the water uptake, which adversely influences the mechanical strength, high amounts of HEMA will result in flexible polymers with inferior qualities(Van Landuyt et al. 2007).
Therefore, it seems that the hydrophilicity of the All-Bond 2 adhesive layer affected the microtensile bond strength values, depending on the thickness of the adhesive layer (Tay & Pashley 2003). This finding suggests that the thickness of 50 μm did not create a high-quality adhesive layer. On the other hand, the thickness of 200 μm could increase the degree of hydrophilicity of the bonding interface. According the manufacturer, the All-Bond 3 bonding resin, which does not contain HEMA, is more hydrophobic than that of All-Bond 2. Hence, the influence of the adhesive layer thickness of the material was minimal.
Although one reason for difference in the microtensile bond strength in All-Bond 2 may be difference in the monomer conversion of the adhesive layers (Yan et al. 2010), polyHEMA is basically a flexible porous polymer even after polymerization (Van Landuyt et al. 2007). As such, high concentrations of HEMA in an adhesive may have deteriorating effects on the mechanical properties of the resulting polymer. HEMA also lowers the vapor pressure of water, and probably also of alcohol. High amounts may therefore hinder good solvent evaporation from adhesive solutions (Pashley et al. 1998).
Like all methacrylates, HEMA is vulnerable to hydrolysis, especially at basic pH, but also at acidic pH. HEMA is very frequently added to adhesives, not only to ensure good wetting, but also because of its solvent-like nature. This property improves the stability of solutions containing hydrophobic and hydrophilic components and will keep ingredients into solution (Van Landuyt et al. 2005). Although, the incorporation of HEMA into dentin adhesives is sometimes indispensable, the amount should be carefully controlled not to make the materials too hydrophilic (Tay & Pashley 2003). According to a previous study (Kim et al. 2014), dentin surface moisture has a crucial effect on the bond strength of resin materials. This issue was not included in this study; further studies are needed.
Ⅳ. CONCLUSION
The influence of adhesive layer thickness on microtensile bond strength to dentin was found to be material-specific. As for All-Bond 2, the relatively thick adhesive layer produced an enhanced resin bonding to dentin compared with the thin adhesive layer. For All-Bond 3, the thickness of the adhesive layer did not significantly affect the microtensile bond strength. These results seem to have relation with the incorporation of the hydrophilic monomer HEMA into the bonding resins.
Acknowledgments
This research was supported by Kyungpook National University Research Fund, 2012(2013, 2014).
References
- Burrow, MF., Inokoshi, S., Tagami, J., (1999), Water sorption of several bonding resins, Am J Dent, 12, p295-298.
-
Cadenaro, M., Breschi, L., Antoniolli, F., et al , (2008), Degree of conversion of resin blends in relation to ethanol content and hydrophilicity, Dent Mater, 24, p1194-1200.
[https://doi.org/10.1016/j.dental.2008.01.012]

-
Di Hipólito, V., Rodrigues, FP., Piveta, FB., et al , (2012), Effectiveness of self-adhesive luting cements in bonding to chlorhexidine-treated dentin, Dent Mater, 28, p495-501.
[https://doi.org/10.1016/j.dental.2011.11.027]

-
El Zohairy, AA., De Gee, AJ., Mohsen, MM., Feilzer, AJ., (2005), Effect of conditioning time of self-etching primers on dentin bond strength of three adhesive resin cements, Dent Mater, 21, p83-93.
[https://doi.org/10.1016/j.dental.2003.12.002]

-
Hiraishi, N., Yiu, CK., King, NM., Tay, FR., (2009), Effect of pulpal pressure on the microtensile bond strength of luting resin cements to human dentin, Dent Mater, 25, p58-66.
[https://doi.org/10.1016/j.dental.2008.05.005]

-
Kim, DH., Son, JS., Jeong, SH., Kim, YK., Kim, KH., Kwon, TY., (2015), Efficacy of various cleaning solutions on saliva-contaminated zirconia for improved resin bonding, J Adv Prosthodont, 75, p85-92.
[https://doi.org/10.4047/jap.2015.7.2.85]

-
Kim, YK., Min, BK., Son, JS., Kim, KH., Kwon, TY., (2014), Influence of different drying methods on microtensile bond strength of self-adhesive resin cements to dentin, Acta Odontol Scand, 72, p954-962.
[https://doi.org/10.3109/00016357.2014.926024]

-
Kwon, TY., Bagheri, R., Kim, YK., Kim, KH., Burrow, MF., (2012), Cure mechanisms in materials for use in esthetic dentistry, J Investig Clin Dent, 3, p3-16.
[https://doi.org/10.1111/j.2041-1626.2012.00114.x]

-
Mazzitelli, C., Monticelli, F., Osorio, R., Casucci, A., Toledano, M., Ferrari, M., (2008), Effect of simulated pulpal pressure on self-adhesive cements bonding to dentin, Dent Mater, 24, p1156-1163.
[https://doi.org/10.1016/j.dental.2008.01.005]

-
Pashley, EL., Zhang, Y., Lockwood, PE., Rueggeberg, FA., Pashley, DH., (1998), Effects of HEMA on water evaporation from water-HEMA mixtures, Dent Mater, 14, p6-10.
[https://doi.org/10.1016/S0109-5641(98)00003-7]

-
Rueggeberg, FA., Margeson, DH., (1990), The effect of oxygen inhibition on an unfilled/filled composite system, J Dent Res, 69, p1652-1658.
[https://doi.org/10.1177/00220345900690100501]

-
Suh, BI., (2004), Oxygen-inhibited layer in adhesion dentistry, J Esthet Restor Dent, 16, p316-323.
[https://doi.org/10.1111/j.1708-8240.2004.tb00060.x]

- Suh, BI., Feng, L., Pashley, DH., Tay, FR., (2003), Factors contributing to the incompatibility between simplified-step adhesives and chemically-cured or dual-cured composites. Part III. Effect of acidic resin monomers, J Adhes Dent, 5, p267-282.
- Tay, FR., Pashley, DH., (2003), Have dentin adhesives become too hydrophilic?, J Can Dent Assoc, 69, p726-731.
- Tay, FR., Suh, BI., Pashley, DH., Prati, C., Chuang, SF., Li, F., (2003), Factors contributing to the incompatibility between simplified-step adhesives and self-cured or dual-cured composites. Part II. Single-bottle, total-etch adhesive, J Adhes Dent, 5, p91-105.
-
Van Landuyt, KL., De Munck, J., Snauwaert, J., et al , (2005), Monomer-solvent phase separation in one-step self-etch adhesives, J Dent Res, 84, p183-188.
[https://doi.org/10.1177/154405910508400214]

-
Van Landuyt, KL., Snauwaert, J., De Munck, J., et al , (2007), Systematic review of the chemical composition of contemporary dental adhesives, Biomaterials, 28, p3757-3785.
[https://doi.org/10.1016/j.biomaterials.2007.04.044]

-
Yan, YL., Kim, YK., Kim, KH., Kwon, TY., (2010), Changes in degree of conversion and microhardness of dental resin cements, Oper Dent, 35, p203-210.
[https://doi.org/10.2341/09-174-L]

-
Yip, HK., Tay, FR., Ngo, HC., Smales, RJ., Pashley, DH., (2001), Bonding of contemporary glass ionomer cements to dentin, Dent Mater, 17, p456-470.
[https://doi.org/10.1016/S0109-5641(01)00007-0]


