Expressions of IL-10 and MT-MMP-2 in Human Chronic Periodontitis with Type 2 Diabetes Mellitus
본 연구는 제2형 당뇨병 환자와 비당뇨 환자에서 만성 치주염 부위의 치은 및 건강한 치은에서 항염증사이토카인인 IL-10 및 교원질 분해효소 중 하나인 MT-MMP-2의 발현에 대해 상호 비교 분석함으로서 혈당이 염증진행에 미치는 영향을 밝히고 제 2형 당뇨병 환자에서 치주조직 파괴의 기전을 알아보고자 하였다.
경북대학교병원 치주과 내원환자 중 제2형 당뇨병 환자와 비당뇨 환자 및 치주질환이 없는 건강인 대조군을 대상으로 여러가지 환자요소, 임상 치주상태를 기록하고 , 전신적으로 건강한 환자의 건강한 부위(Group 1), 전신적으로 건강한 환자의 만성 치주염 부위(Group 2), 제2형 당뇨병 환자의 만성 치주염 부위 (Group 3)에서 각각 변연치은을 채득하고 액화질소에 급속 동결하였다. Western blotting을 이용하여 각 조직 내 IL-10및 MT-MMP-2의 발현을 관찰하고, densitometer를 이용하여 상대적 발현을 정량, 각 조직의 β-actin을 표준화하여 평균치를 ANOVA 분석으로 통계처리하여 다음과 같은 결과를 얻었다.
IL-10은 전신적으로 건강한 환자의 치은조직 및 단순 만성 치주염 환자의 치은조직보다 제 2형 당뇨병 환자의 염증성 조직에서 가장 적게 발현되는 양상을 보였다. 전신적으로 건강한 환자의 정상조직에서 발현이 가장 높게 나타났으며 그 차이가 통계학적으로 유의하였다. MT-MMP-2는 단순 만성 치주염 환자와 제 2형 당뇨병을 동반한 만성 치주염 환자에서 전신적으로 건강한 환자에서보다 증가하는 양상을 보였으며 통계학적으로 유의하였다.
결론적으로 IL-10과 MT-MMP-2는 단순 만성 치주염과 제2형 당뇨병 환자의 만성 치주염의 진행에 관여하는인자로 보이며 염증상태와 치조골 흡수를 나타내는 지표로 응용할 수 있으리라 사료된다.
Keywords:
chronic periodontitis, interleukin-10 (IL-10), MT-MMP-2, type 2 diabetes mellitusINTRODUCTION
Periodontal disease is a microbe-induced chronic inflammatory condition that leads to gingival inflammation, periodontal tissue destruction, and alveolar bone loss. The histopathological characteristics of periodontitis include periodontal pocketing, location of junctional epithelium apical to the cemento-enamel junction, loss of collagen fibers, numerous polymorphonuclear leukocytes in the junctional and pocket epithelium, and a dense inflammatory cell infiltrate with plasma cells, lymphocytes, and macrophages (Seymour & Greenspan, 1979; Socransky & Haffajee, 1992)
Diabetes mellitus (DM) is a clinically and genetically heterogeneous group of metabolic disorders manifested by abnormally high levels of glucose in the blood. The hyperglycemia is the result of a deficiency of insulin secretion caused by pancreatic β-cell dysfunction (Type 1 DM) or resistance to the action of insulin in liver and muscle (Type 2 DM), or a combination of these (Nishimura F et al., 1998; Brian L et al., 2007). Type 2 DM is the more common form and occurs mainly in adults. It is frequently reported that type 2 DM comprises approximately 90% of all the cases of DM in the population.
The association between DM and periodontitis has long been discussed. Engebretson et al. (1999) reported that DM increases the risk for the development of periodontitis. Soskolne et al.(1998) also demonstrated that periodontal disease is more prevalent and more severe in DM patients. This suggests that some DMinduced metabolic alterations serve to diminish host resistance to periodontal breakdown. Hyperglycemia has the potential to alter the local environment in the periodontal pocket. Human gingival fibroblasts produce decreased amounts of collagen and glycosaminoglycans in high-glucose environments(Willershausen-Zonnchen B et al., 1991). DM increases glucose concentration in the gingival crevicular fluid, and decreases the salivary levels of epidermal growth factor, which plays an important role in wound healing.
The immune response against periodontopathic bacteria is regulated by the balance between cytokines produced by T helper 1 (Th1) and T helper 2 (Th2) cells (Gemmell E, Seymour GJ, 2004). Cytokines, small protein released by cells, are the most common signals in immune regulation, and the cytokine concentration in the gingival tissues is related to periodontal conditions (Fokkema SJ et al., 2003). The typical secretory products of Th1 cells are IL-2, IL-12, TNF-β, and IFN- γ; those of Th2 cells are IL-4, IL-5, IL-6, IL-10 and IL-13.
IL-10 is a 35 kD polypeptide produced by B cells, T-cell, and macrophages. While it plays a major role in suppressing immune and inflammatory responses, it is also a potent growth and differentiation factor for activated human B cells (Moore WEC et al, 1982). Furthermore, IL-10 is known to have a protective role against the lethal effects of LPS in vivo(Leon LR et al., 1998). IL-10 also increases the recruitment of macrophages and neutrophils, and inhibits cancer growth by hampering tumour angiogenesis and invasiveness through induction of metalloproteinase (MMP) inhibitors(Stearns ME et al., 1999). Indeed, interleukin-10 may be critical in controlling the balance between Th 1 and Th 2 cells in chronic periodontitis, whereby an excess of IL-10 may shift the balance in favor of a T helper 2 response, whereas a deficiency may lead to increased IL-1 production and increased tissue destruction(Cullinan MP et al., 2008).
MMPs is composes of at least 23 related zincdependent endopeptidases that are able to degrade extracellular matrix proteins(Nagase & Woessner, 1997). MMPs perform multiple roles in the host response to the progression of infection, facilitating leukocyte recruitment, cytokine and chemokine processing and matrix remodeling(Ryan & Golub, 2000). MMPs are tightly regulated by the levels of active enzymes and their inhibitors, the tissue inhibitors of metalloproteinases (TIMPs). A relationship has been established between collagen degradation during advanced periodontitis and the balance between MMPs and TIMPs. MMPs can be classified into at least five subgroups including collagenases, gelatinases, stromelysin, membrane-type collagenases, and matrilysins according to their structural and functional characteristics(Gulnur E et al., 2006). Membrane- type matrix metalloproteinase, including MT-MMP-1 (also designated MMP-14), MT-MMP-2(also designated MMP-15), MT-MMP-3 (also designated MMP-16), and MT-MMP-4 (also designated MMP-17) are type 1 membrane proteins that function to activate other MMPs. MT-MMP-2 activation appears to be mediated by members of the proprotein convertase family, may be involved in the regulation of ECM turnover. d’Ortho et al.(1997) suggested that MT-MMP-2 could act in concert with activated gelatinase A/MMP2 to degrade matrix components such as fibronectin and tenasin.
Cytokines are considered to play a key role in the inflammation process. In inflammatory response with bone resorption, the role and interactions of IL-10 and MT-MMP-2 are not clear, and their relative contribution to the pathogenesis of periodontitis and alveolar bone resorption is not entirely established yet. The purpose of this study was to observe and quantify the expression of IL-10 and MT-MMP-2 in the gingival tissue of patients with type 2 DM and systemically healthy adults with chronic periodontitis.
MATERIALS AND METHODS
1. Study population and tissue sampling
The study population consisted of 12 patients with 12 healthy individuals (Group 1), 12 patients with chronic periodontitis(Group 2) and type 2 diabetes and chronic periodontitis (Group 3). Marginal gingival tissue samples were obtained by internal bevel incision at the time of periodontal surgery (including surgical crown lengthening) or tooth extraction and informed consent was obtained from all of the participants before the surgery. This study was approved by the Ethical Committee of Clinical Experiments, Kyungpook National University (74005-1119).
Clinical criteria of gingiva (Sulcus bleeding index value, probing depths) and radiographic evidences of bone resorption, each gingival sample was divided into the three groups(Joo SD, Lee JM., 2007). Group 1 (normal, n=12) is clinically healthy gingiva without bleeding and no evidence of bone resorption or periodontal pockets, obtained from systemically healthy 12 patients. Group 2 (chronic periodontitis, n=12) is inflamed gingiva from patients with chronic periodontitis. The diagnosis of chronic periodontitis was established on the basis of clinical and radiographic criteria (bone resorption) according to the classification system for periodontal disease and condition. All patients of group 2 were systemically healthy and had more than one periodontal pockets ≥5 mm and at least one pocket with ≥4mm loss of attachment. All gingival samples were obtained from the teeth with probing depth ≥5 mm, swelling of the marginal gingiva, and bleeding corresponding to gingival sulcus bleeding indexes 3 according to Mühlman and Son. Group 3 (chronic periodontitis & type 2 DM, n=12) is inflamed gingiva from patients with chronic periodontitis associated with type 2 diabetes. Patients in group 2 & 3 have similar periodontal condition, but patients in group 2 were systemically healthy and patients in group 3 had type 2 DM with treatment. Patients in group 3 were diagnosed type 2 DM since 6 months and showed above 200 mg/dl blood glucose level in postprandial 2 hours. Gingival sample were obtained by similar way described above.
Following surgery, excised tissue specimens were immediately placed on liquid nitrogen and subsequently frozen (-70℃).
2. Protein isolation and western blotting
For western blotting, as previously described technique by Kim et al. (Kim DH et al., 2006) frozen tissues were homogenized in RIPA lysis buffer (10 mM EDTA, 0.15M NaCl) with 1:30 diluted protease inhibitor cocktail (Roche, Mannheim, Germany) according to Cho et al. (2000)’s method. The lysates were sonicated three times for 10 seconds and centrifuge at 12,000g for 15 minutes. Protein concentrations of supernant were routinely determined by a Braford protein asssay (Quick StartTM, BIO-RAD, Hercules, USA) using bovine serum albumin(BSA) as standard.
Lysates were boiled in SDS samples buffer (1M Tris-Cl (pH6.8), 40% glycerol, 8% SDS, 2% mercapto-ethanol, 0.002% Bromophenole blue). Prepared samples were separated by 15% sodium dodecyl sulfate (SDS) - polyacrylamide gels and transferred to a polyvinylidene difluride membrane.
The membranes were subsequently blocked in Tris -buffered saline (TBS) containing 5% powdered milk and 1% BSA for 1 hour, and then incubated with polyclonal anti-IL-10 and anti-MT-MMP-2 antibody (Prepared in rabbit and goat, diluted 1:1,000 in TBS, respectively, Sigma-Aldrich, Inc. USA) for 1.5 hours at room temperature.
The membranes were washed (five times for 5 minutes with Tween 20) and incubated with a horseradish peroxidase (HRP) - conjugated goat anti-rabbit and dongkey anti-goat secondary antibody for anti-IL-10 and anti-MT-MMP-2 (diluted 1: 2,000 in TBS) antibody for 1 hour at room temperature. After additional washing (five times for 5 minutes with Tween 20), the western blot procedure was completed with an ECL Plus development kit (Amsterdam, Beckinghamshire, U.K.)
The quantification analysis of IL-10 and MT-MMP-2 expression was performed using a densitometer (Scion Image β 4.02, Scion Corporation, Frederick, USA). After normalization to β-actin (Abcam, Edinburgh, U.K.) in each sample, level of IL-10 and MT-MMP-2 were expressed as a ratio of IL-10 or MT-MMP-2/β-actin and the differences of density between three groups were determined.
3. Statistical analysis of the western blot results
All data were presented as means ± standard deviation and results were statistically analyzed. The IL-10 and MT-MMP-2 levels among the three groups were compared using one-way ANOVA followed by Tukey’s test. A p value < 0.05 was considered to be statistically significant.
RESULTS
Both the chronic periodontitis group & the chronic periodontitis with type 2 DM group showed the expression of IL-10 and MT-MMP-2 in all samples. To compare IL-10 expression levels in human gingiva with chronic periodontitis with or without associated to Type 2 DM, IL-10 specific antibodies were used to detect the cytokine in the tissues (Figure. 1A, B). Representative western blot data (Figure. 1A) detected about a 35 kD molecular weight of IL-10 in all three groups. The expression levels of β-actin were also measured by anti-β-actin specific western blot analysis. In order to quantify the level of IL-10 expression in the groups, the expression levels of IL-10 in each sample were measured by densitometer. Then IL-10 expression levels were normalized by β-actin (ratio of IL-10/β-actin). The levels of gingival normalized IL-10 expression were given in Table 1., and summarized as a graph in Figure. 1B.
There was a significant difference between Group 1 and Group 3. But there was no statistically significant difference (p<0.05) between Group 1 and Group 2, and between Group 2 and Group 3.
The comparison of MT-MMP-2 expression levels were also made by western blot analysis using MT-MMP-2 specific antibody (Figure. 2A). The levels of MT-MMP-2 expression which detected molecular weights 64 kD were also quantified with β-actin normalization (Figure. 2B). There was a significant difference between Group 1 and Group 2, and between Group 1 and Group 3 (p<0.05).
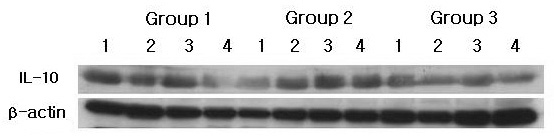
IL-10 western blot analysis showing 4 representative samples in each group. IL-10 levels were quantified on the basis of β-actin levels. IL-10 corresponding to molecular weight 35 kD was shown to be expressed in all samples including healthy gingiva, and the expression levels of IL-10 were decreased in order of Group 1, Group 2 and Group 3. Group 1 : healthy gingiva from systemically healthy individuals Group 2 : inflamed gingiva from patients with chronic periodontitisGroup 3 : inflamed gingiva from patients with chronic periodontitis and type 2 DM
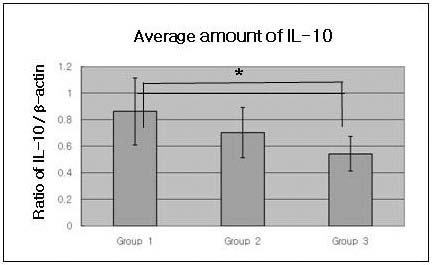
Graphics showing the average amounts (ratio of IL-10/β-actin) and standard deviation of IL-10 level in Groups 1, 2 and 3. In the healthy gingival tissues (Group 1), the levels of IL-4 were significantly increased as compared to Group 2 and Group 3 (p<0.05)Group 1 : healthy gingiva from systemically healthy individualsGroup 2 : inflamed gingiva from patients with chronic periodontitisGroup 3 : inflamed gingiva from patients with chronic periodontitis and type 2 DM* Significant difference between Group 1 and Group 3 (p<0.05).
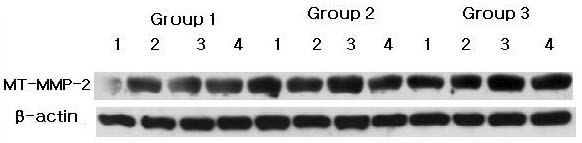
MT-MMP-2 western blot analysis showing 4 representative samples in each group. MT-MMP-2 levels were quantified on the basis of β-actin levels. MT-MMP-2 corresponding to molecular weight 64 kD was shown to be expressed in all samples including healthy gingiva. The expression levels of MT-MMP-2 increased in order of Group 1, Group 2 and Group 3.Group 1 : healthy gingiva from systemically healthy individualsGroup 2 : inflamed gingiva from patients with chronic periodontitisGroup 3 : inflamed gingiva from patients with chronic periodontitis and type 2 DM

Graphics showing the average amounts (Ratio of MT-MMP-2/β-actin) and standard deviation of MT-MMP-2 level in Groups 1, 2 and 3. In the inflamed gingiva (with or without type 2 DM, Group 2 and Group 3), the levels of MT-MMP-2 were higher than those in healthy gingiva.Group 1 : healthy gingiva from systemically healthy individualsGroup 2 : inflamed gingiva from patients with chronic periodontitisGroup 3 : inflamed gingiva from patients with chronic periodontitis and type 2 DM+ Significant difference between Group 1 and Group 2 (p<0.05).* Significant difference between Group 1 and Group 3 (p<0.05).
DISCUSSION
An association between diabetes and periodontitis is a widely accepted phenomenon that has been shown in numerous studies both for Type 1 and 2 DM. The majority of cllinical and epidemiological evidence demonstrates that individuals with diabetes tend to have a higher prevalence and more severe progressing forms of periodontitis than non-diabetics(Graves DT et al., 2006). It was shown that diabetes upregulates the production of inflammatory cytokines and chemokines, leading to increased inflammation, tissue damage, and apoptosis in patients who have periodontitis (Hempel L et al., 1995). In addition, gingival fibroblasts from diabetic patients synthesize less collagen compared to non-diabetic subjects.
The immune response against periodontopathic bacteria is regulated by the balance between cytokines produced by T helper 1 (Th1) and T helper 2 (Th2) cells. Th1 cells regulate a cell-mediated-type immune response, and Th2 cells regulate a humoral-type immune response. In addition, each subset can regulate the function of the other. The typical secretory products of Th1 cells are interleukin(IL)-2, IL-12, TNF-β, and IFN-γ; those of Th2 cells are IL-4, IL-5, IL-6, IL-10 and IL-13. For example, during the generation of a primary Th1 response, IFN-γ acts as a positive regulator by selectively inducing Th1 differentiation through the increased transcription of T-bet, which results in enhanced IL-12 responsiveness and suppressed Th2 lineage commitment (Mullen AC et al., 2001). Evidence of the role of cytokines produced by resident and inflammatory cells during inflammation is well established. The purpose of this study was to quantify and compare the expression of IL-10 and MT-MMP-2 in the gingival tissue of the patients with chronic periodontitis associated to type 2 DM, in order to understand the contribution of these proteins to periodontal destruction in type 2 DM patients. IL-10 is a 35-40 kD polypeptide produced by B cells, T-cell, and macrophages. Regulatory roles of IL-10 on the immune response have been well demonstrated. IL-10 is a key regulatory cytokine that has significant effects on both the innate and adaptive immune responses. Production of cytokines, such as IFN-γ, IL-6, and IL-8, is supressed by IL-10(Yamazaki K et al., 2001). IL-10, an anti-inflammatory cytokine, plays a role in periodontitis by inhibiting synthesis of proinflammatory cytokines such as IL-1, -2, -6, and -8, TNF-α, IFN-γ, and stimulating protective antibody production(Still K et al., 2000; Scarel-Caminaga RM et al., 2004). In this study the quantitative analysis of the IL-10 level showed that IL-10 expression was rather decreased in inflamed gingiva associated with type 2 DM as compared to healthy gingiva, and the difference was statistically significant (p<0.05). Shin et al.(2010) also reported that IL-4 expression was rather decreased in inflamed gingiva associated to type 2 DM compared to healthy gingiva of systemically healthy patients. On the other hand, Park et al.(2007) demonstrated that IL-6 expression was higher in inflamed gingiva with chronic periodontitis associated to type 2 DM. It suggests that where a lack of IL-10 may fail to protect the host from excess tissue damage while high levels suppress immune response. This result indicates that IL-10 inhibits inflammatory response in disease progression in chronic periodontitis with type 2 DM patients and plays a role in decreased inflammatory response with bone resorption in patients with this systemic disease.
The pro-inflammatory cytokines stimulate cells of the host to produce a number of MMPs, which are eventually responsible for degradation of periodontal connective tissues in the pathogenesis of periodontitis. It is now recognized that during active periodontitis, degradation of gingival tissue is due in part to MMPs expressed in situ by inflammatory cells. Degradation of the extracellular matrix is thought to occur only when activated enzyme levels exceed the levels of active inhibitors. Ejeil et al.(2003) suggested that MMP-2 and MMP-9, mainly cleaving type IV collagen, are also believed to play important roles in tissue destruction in periodontitis. In this study, the quantitative analysis of MT-MMP-2 levels showed that MT-MMP-2 expression was rather increased in inflamed gingiva with or without type 2 DM as compared to healthy gingiva, and the difference was statistically significant (p<0.05). Since MT-MMP-2 have been found to be expressed in chronic periodontitis, the disruption of basement membrane component is thought to be an important step. Korostoff et al.(2000) reported that host-derived MMPs are thought to play a prominent role in the tissue destruction in the progression of periodontitis. The elevated level of MMPs released from host cells may be due to the stimulation of pro-inflammatory cytokines and bacteria. Our data also demonstrated that the total amount of cytokine MTMMP- 2 in active sites in patients with the progression of periodontitis is significantly higher than in inactive sites. The pro-inflammatory cytokines stimulates cells of the host to produce a number of MMPs, which are eventually responsible for degradation of periodontal connective tissues in pathogenesis of periodontitis.
In conclusions, this study demonstrated that MT-MMP-2 expression levels in human gingival tissue have a positive correlation with the bone resorption process in inflamed tissue and inflamed tissue associated with type 2 DM. This suggests that MT-MMP-2 may be involved in the alveolar bone resorptive process of periodontal inflammation associated with type 2 DM. On the other hand, the tissue with chronic periodontitis associated with type 2 DM showed significantly decreased IL-10 levels compared to healthy gingiva and non-diabetic inflamed gingiva. This suggests that IL-10 may be involved in the retrogression of periodontal inflammation associated with type 2 DM.
Finally, more studies are needed to investigate the effect and interrelationship between IL-10, MT-MMP-2 and other cytokines that affect the progression of periodontal disease at a higher level. Further studies will contribute to the development of disease diagnosis methods and treatment modalities.
References
- L Brian, L Mealey, L Gloria, Ocampo, Diabetes mellitus and periodontal disease, Periodontol 2000, (2007), 44, p127-153.
-
JY Cho, S Xing, X Liu, TLF Buckwalter et al, Expression and activity of human Na+/I-symporter in human glioma cells by adenovirus-mediated gene delivary, Gene Therapy, (2000), 7, p740-749.
[https://doi.org/10.1038/sj.gt.3301170]

-
MP Cullinan, B Westerman, SM Hamlet et al, Progression of periodontal disease and interleukin- 10 gene polymorphism, J Periodont Res, (2008), 43, p328-333.
[https://doi.org/10.1111/j.1600-0765.2007.01034.x]

-
MP d'Ortho, H Will, S Atkinson et al, Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases, Eur J Biochem, (1997), 250, p751-757.
[https://doi.org/10.1111/j.1432-1033.1997.00751.x]

-
L Ejeil, S Igondjo-Tchen, S Ghomrasseni, B Pellat, G Godeau, B Gogly, Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in healthy and diseased human gingiva, J Periodontol, (2003), 74, p188-195.
[https://doi.org/10.1902/jop.2003.74.2.188]

- SP Engebretson, E Lalla, IB Lamster, Periodontitis and systemic disease, N Y State Dent J, (1999), 65, p30-32.
-
SJ Fokkema, BG Loos, C de Slegte et al, Increased release of IL-12p70 by monocytes after periodontal therapy, J Clin Periodontol, (2003), 30, p1091-1096.
[https://doi.org/10.1046/j.0303-6979.2003.00435.x]

-
E Gemmell, GJ Seymour, Immunoregulatory control of Th1/Th2 cytokine profiles in periodontal disease, Periodontol 2000, (2004), 35, p21-41.
[https://doi.org/10.1111/j.0906-6713.2004.003557.x]

-
DT Graves, R Liu, M Alikhani, H Al-Mashat, PC Trackman, Diabetes-enhanced inflammation and apoptosis – Impact on periodontal pathology, J Dent Res, (2006), 85, p15-21.
[https://doi.org/10.1177/154405910608500103]

- E Gulnur, T Taina, M Paivi, M Marko, S Timo, A Gul, Gingival Crevicular Fluid Matrix Metalloproteinase (MMP)-7, Extracellular MMP Inducer, and Tissue Inhibitor of MMP-1 Levels in Periodontal Disease, J Periodontol, (2006), 77, p2040-2050.
-
L Hempel, D Korholz, H Bonig et al, Interleukin- 10 directly inhibits interleukin-6 production in T-cells, Scand J Immunol, (1995), 41, p462-466.
[https://doi.org/10.1111/j.1365-3083.1995.tb03593.x]

- SD Joo, JM Lee, The comparison of inflammatory mediator expression in gingival tissues from human chronic periodontitis patients with and without type 2 diabetes, J Korean Acad Periodontol, (2007), 37, p353-369.
- DH Kim, JW Park, JM Lee et al, The comparison of MMP-2, MMP-9 and tumor necrosis factor-α expressions in human gingival with chronic periodontitis with or without associated to type 2 diabetes mellitus, J Korean Aca Periodontol, (2006), 36, p409-426.
- M Korostoff, F Wang, P Sarment, C Stewart, S Feldman, C Billings, Analysis of in situ protease activity in chronic adult periodontitis patients: expression of activated MMP-2 and a 40 kDa serine protease, J Periodontol, (2000), 71, p353-360.
-
LR Leon, W Kozak, MJ Kluger, Role of IL-10 in inflammation. Studies using knockout mice, Ann NY Acad Sci, (1998), 856, p69-75.
[https://doi.org/10.1111/j.1749-6632.1998.tb08314.x]

- WEC Moore, LV Holdeman, RM Smibert, DE Hash, JA Burmeister, RR Ranney, Bacteriology of severe periodontitis in young adult humans, Infect Immun, (1982), 38, p1137-1148.
-
AC Mullen, FA High, AS Hutchins et al, Role of T-bet in commitment of Th1 cells before IL-12-dependent selection, Science, (2001), 292, p1907-1910.
[https://doi.org/10.1126/science.1059835]

- H Nagase, JF Jr Woessner, Matrix metalloproteinases, Biol Chem, (1997), 378, p151-160.
-
F Nishimura, K Takahashi, M Kurihara, S Takashiba, Y Murayama, Periodontal disease as a complication of diabetes mellitus, Ann Periodontol, (1998), 3, p20-29.
[https://doi.org/10.1902/annals.1998.3.1.20]

- JW Park, JM Lee, The comparison of IL-6, elastase and α1-PI expressions in human chronic periodontitis with type 2 diabetes mellitus, J Korean Acad Periodontol, (2007), 37, p325-338.
-
ME Ryan, LM Golub, Modulation of matrix metalloproteinase activities in periodontitis as a treatment strategy, Periodontol 2000, (2000), 24, p226-238.
[https://doi.org/10.1034/j.1600-0757.2000.2240111.x]

-
RM Scarel-Caminaga, PC Trevilatto, AP Souza, RB Brito, LE Camargo, SR Line, Interleukin 10 gene promoter polymorphisms are associated with chronic periodontitis, J Clin Periodontol, (2004), 31, p443-448.
[https://doi.org/10.1111/j.1600-051X.2004.00500.x]

-
GJ Seymour, JS Greenspan, The phenotypic characterization of lymphocyte subpopulations in established human periodontal disease, J Periodont Res, (1979), 14, p39-46.
[https://doi.org/10.1111/j.1600-0765.1979.tb00216.x]

-
DS Shin, JW Park, JY Suh, JM Lee, The expressions of inflammatory factors and tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) in human chronic periodontitis with type 2 diabetes mellitus, J Periodontal Implant Sci, (2010), 40, p33-38.
[https://doi.org/10.5051/jpis.2010.40.1.33]

-
SS Socransky, AD Haffajee, The bacterial etiology of destructive periodontal disease: Current concepts, J Periodontol, (1992), 63, p322-331.
[https://doi.org/10.1902/jop.1992.63.4s.322]

-
WA Soskolne, Epidemiological and clinical aspects of periodontal diseases in diabetics, Ann Periodontol, (1998), 3, p3-12.
[https://doi.org/10.1902/annals.1998.3.1.3]

-
K Still et al, Localization and Quantification of mRNA for Matrix Metalloproteinase-2 (MMP-2) and Tissue Inhibitor of Matrix Metalloproteinase-2 (TIMP-2) in Human Benign and Malignant Prostatic Tissue, Prostate, (2000), 42, p18-25.
[https://doi.org/10.1002/(SICI)1097-0045(20000101)42:1<18::AID-PROS3>3.0.CO;2-A]

- ME Stearns et al, Interleukin-10 (IL-10) inhibition of primary human prostate cell-induced angiogenesis: IL-10 stimulation of tissue inhibitor of metalloproteinase- 1 and inhibition of matrix metalloproteinase (MMP)-2/MMP-9 secretion, Clin Cancer Res, (1999), 5, p189-196.
-
B Willershausen-Zonnchen et al, Influence of high glucose concentrations on glycosaminoglycan and collagen synthesis in cultured human gingival fibroblasts, J Clin Periodontol, (1991), 18, p190-195.
[https://doi.org/10.1111/j.1600-051X.1991.tb01132.x]

-
K Yamazaki et al, Interleukin-10 gene promoter polymorphism in Japanese patients with adult and early-onset periodontitis, J Clin Periodontol, (2001), 28, p828-832.
[https://doi.org/10.1034/j.1600-051x.2001.028009828.x]