The Expressions of Xanthine oxidase and Malondialdehyde in Chronic Periodontitis with Hypertension
The purpose of this study was to quantify the expressions of XO and MDA in the gingival tissues of the chronic periodontitis patients associated with HTN. Xanthine oxidase (XO) generates reactive oxygen species. Malondialdehyde (MDA) is one of many low molecular weight end-products of LPO and is the most often measured as an index of peroxidation. Depending on the patient's systemic condition and clinical criteria of gingiva, each gingival sample was divided into three groups. Group 1 is clinically healthy gingiva. Group 2 is inflamed gingiva from patients with chronic periodontitis. Group 3 is inflamed gingiva from patients with chronic periodontitis associated with HTN. The expression levels of XO increased in order of group 1, group 2 and group 3, and significantly increased in group 3 as compared to group 1. The expression levels of MDA increased in order of group 1, group 2 and group 3. MDA expression was significantly higher in group 2 than in group 1, and the quantitative analysis of MDA level was significantly higher in group 3 than in group 1. In conclusion, this study demonstrated that the expression levels of XO and MDA might be inflammatory and bone resorption marker in periodontal inflammed tissue. It is assumed that hypertension may be associated with the progression of periodontal inflammation and alveolar bone resorption.
Keywords:
chronic periodontitis, Xanthine oxidase, Malondialdehyde, HypertensionINTRODUCTION
Periodontitis is a chronic inflammatory disease caused by oral bacterial infection (Page & Kornman, 1997). When stimulated by bacterial pathogens, host immune response is accompanied by increase in cytokines expression such as IL-8 and TNF-α, leading to elevated numbers and activity of polymorphonuclearleukocyte (PMN). As a result of stimulation by bacterial pathogens, PMN produces the reactive oxygen species (ROS) via the respiratory burst, during the process of phago-cytosis (Sculley & Langley-Evans, 2002).
Excessive production of ROS in PMN is one of the pathologic features in the periodontal lesion (Ekuni et al., 2008), and it leads to damage of the periodontal tissue by oxidizing DNA, lipids, and proteins (Chapple et al., 2007).
ROS is a collective term, which encompasses not only true free radicals but also other reactive species which are capable of radical formation in the intraand extracellular environments. Excess production of ROS and the resultant oxidative stress contribute significantly to tissue damage in many diseases in over 100 disorders such as rheumatoid arthritis (RA), diabetes, AIDS and cancer (Curnutte & Babior, 1987; Halliwell B, 1996). Oxygen-derived free radicals include superoxide (O2-), hydroxyl (OH) and nitric oxide (NO), and non-radical derivatives of oxygen include hydrogen peroxide (H2O2) and hypochlorous acid (HOCL) (Chapple & Matthews, 2007; Halliwell B, 2000; Waddington et al., 2000).
Xanthine oxidase (XO), a form of xanthine oxidoreductase, that generates reactive oxygen species (Ardan et al., 2004), and an enzyme that catalyzes the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid (Harrison R, 2002). XO generated ROS are implicated in both tissue structural damage and cell signaling interference, and can cause lipid peroxidation (LPO), resulting in disruption of membrane architecture and lysosomal enzyme release (Weiss SJ, 1986), and DNA and amino acid oxidation, causing genetic mutations and enzyme dysfunction or proteolysis (Turko & Murad, 2002). Dysfuction of proteins due to ROS has been implicated in the pathophysiology of several cardiovascular diseases (CVD), including autoimmune myocarditis, HTN, and heart failure (Kehrer JP, 2000), although this remains controversal. In regard to XO, oxidative injury is often achieved via the byproducts of O2- and H2O2 generation.
ROS can induce lipid peroxidations (LPO) having effects on cells (Ornoy A, 2007). Redundant production of LPO can result in oxidative stress and consequently, damage to cell integrity. Because LPO results from oxidative stress, numerous markers have been used to monitor this process.
Malondialdehyde (MDA) is one of many low molecular weight end-products of LPO and is the most often measured as an index of peroxidation, which has numerous deleterious effects on biological systems (Richter C, 1987; Matés et al., 1999). An increased free radicals causes overproduction of MDA that reacts with DNA, cause a mutagenic transformation within DNA. In addition, MDA interact with several functional groups on proteins, altering their chemical behavior and possibly contributing to carcinogenesis and mutagenesis. Several pathologic disease have been studied from an oxidative viewpoint in recent years and MDA has been used as a common oxidative stress biomarker. Gönenç et al. (2001) found higher levels of plasma MDA in subjects affected by breast or lung cancer. MDA plasma levels were found to be higher in non-insulin dependent DM subjects than in healthy controls in the previous study (Dierckx et al., 2003). CVD have also been related to free radical-mediated mechanisms and to LPO. Polidori et al. (2002) found higher levels of antioxidants and lower levels of MDA in healthy subjects when compared in a case-control study with congestive heart failure patients.
HTN is a major contributor to the development of renal failure, CVD, and stroke and has been the object of many studies due to its high prevalence and high impact on morbidity and mortality (Kim et al., 1994). Among the factors associated with the development of hypertension, an extremely important, complex and current one is the participation of ROS in its pathogenesis. ROS influence vascular, renal, and cardiac function and structure by modulating cell growth, contraction/dilatation, and inflammatory responses via redox-dependent signaling pathways. Redox processes, mainly through the enzymes NAD(P)H oxidase, XO, and endothelial nitric oxide synthase (eNOS), act not only by increasing the production of O2- but also through mechanical forces that stimulate O2- production (Briones & Touyz, 2010).
The association between periodontal disease and systemic diseases such as DM, CVD and HTN has recently received much attention. The changes in the host response as a consequence of various diseases can affect the severity and prognosis of periodontitis. Over the past few years, several epidemiologic studies examined the potential association between periodontitis and HTN (Perlstein & Bissada, 1977). Possible explanation of association between periodontitis and HTN is that vascular changes in gingival tissue with HTN contribute to an increase of inflammation and alveolar bone resorption in gingival tissue (Fabio et al., 2003).
There are few data related with HTN and periodontal disease, despite the high prevalence of HTN in the general population and its leading prognostic importance. So far, it has been reported that somewhat controversal results about the roles and interactions of ROS in the periodontal tissues. However the relation between ROS and periodontitis are not entirely understood yet. Moreover, few studies have been simultaneously analyzed ROS in periodontitis with or without hypertension.
The purpose of this study was to quantify the expressions of MDA and XO in the gingival tissues of chronic periodontitis patients associated to HTN.
MATERIALS AND METHODS
1. Study population and Tissue sampling
Study population was consisted of 16 patients with hypertension and chronic periodontitis, 16 patients with chronic periodontitis, and 16 healthy individuals. Marginal gingival tissue samples were obtained by internal bevel incision at the time of periodontal surgery (including surgical crown lengthening) or tooth extraction and informed consent was obtained from all of the participants before the surgery. This study was approved by the Ethical Committee of clinical experiments, Kyungpook National University Hospital.
According to the patient's systemic condition (age, sex, blood pressure, obesity and smoking), the clinical criteria of gingiva (Sulcus bleeding index value, probing depth) and radiographic evidences of bone resorption, each gingival sample was divided into the three groups.
Group 1 (control) is clinically healthy gingiva without bleeding and evidence of bone resorption or periodontal pockets, obtained from systemically healthy 16 patients.
Group 2 (chronic periodontitis) is inflamed gingiva from patients with chronic periodontitis. The diagnosis of chronic periodontitis was established on the basis of clinical and radiographic criteria (bone resorption) according to the classification system for periodontal disease and condition. All patients of group 2 were systemically healthy and had more than one periodontal pockets ≥5 mm and at least one pocket with ≥5 mm loss of attachment. All gingival samples were obtained from the teeth with probing depth ≥5 mm, swelling of the marginal gingiva, and bleeding corresponding to gingival sulcus bleeding indexes above 3 according to Mühlmann and Son (1971).
Group 3 (chronic periodontitis with hypertension) is inflamed gingiva from patients with chronic periodontitis associated with hypertension. Patients in group 3 were diagnosed hypertension since 6 months and showed systolic pressure greater than 140 mmHg or diastolic pressure greater than 90 mmHg without medication. Patient characteristics are presented in Table 1.
After surgery, excised tissue specimens were immediately placed on liquid nitrogen and subsequently frozen at -70℃.
2. Protein Isolation and Western blotting
For Western blotting, as previously described technique by Kim et al. (2011) and Lee et al. (2010), frozen tissues were homogenized in RIPA lysis buffer (10 mM EDTA, 0.15M NaCl) with 1:30 diluted protease inhibitor cocktail (Roche, Germany) (Cho et al.. 2000). The lysates were sonicated 3 times for 10 seconds and centrifuged at 12,000g for 15 minutes. Protein concentrations of supernatant were routinely determined by a Braford protein asssay (Quick Start, BIO-RAD, USA) using BSA as standard. Lysates were boiled in SDS samples buffer (1M Tris-Cl (pH6.8), 40% glycerol, 8% SDS, 2% mercapto-ethanol, 0.002% Bromophenole blue). Prepared samples were separated by 15% sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to a polyvinylidene difluride (PVDF) membrane. The membranes were subsequently blocked in Trisbuffered saline (TBS) containing 5% powdered milk and 1% BSA for 1 hour, and then incubated with polyclonal anti-XO antibody (diluted 1:1,000 in TBS, sc-22006, Santa Cruz Biotechnology, Inc. USA) and anti-MDA antibody (diluted 1:1,000 in TBS, sc-130087, Santa Cruz Biotechnology, Inc. USA) for 1.5 hours at room temperature.
The membranes were washed (five times for 5 minutes with Tween 20) and incubated with a horseradish peroxidase (HRP)-conjugated donkey anti-goat IgG-HRP secondary antibody (diluted 1:2,000 in TBS. sc-2020, Santa Cruz Biotechnology, Inc. USA) for anti-XO antibody and anti-MDA antibody for 1 hour at room temperature. After additional washing (five times for 5 minutes with Tween 20), the Western blot procedure was completed with an ECL Plus development kit (Amsterdam, Beckinghamshire, U.K.)
The relative quantification analysis of XO and MDA expression was performed using a densitometer (Image Gauge V 3.46, Koshin Graphic Systems, Fuji Photo Film Co., Japan). After normalization to β-actin (Abcam®, U.K.) in each sample, levels of XO and MDA were expressed as a ratio of XO and MDA/β -actin and the differences of density between 3 groups were determined.
3. Statistical Analysis of the Western blot results
All data were presented as means ± standard deviation and results were statistically analyzed. The XO and MDA levels were compared using one way ANOVA followed by Tukey test. P value < 0.05 was considered to statistically significant.
RESULTS
Marginal gingival tissues from three groups showed the expression of XO and MDA in all samples.
The comparison of XO expression levels was also studied by Western blot analysis using a XO specific antibody which detected about 150 kDa molecular weight of XO in all three groups (Fig. 1). The normalized levels of individual gingival XO expression (ratio of XO/β-actin) were given in Table 2 and summarized as a graph in Fig. 2. The mean value of XO expression was 0.652±0.178 for group 1, 0.741±0.195 for group 2, 0.856±0.199 for group 3. There was a significant difference between group 1 and group 3. However, there was no statistically differences between group 1 and group 2, between group 2 and group 3.
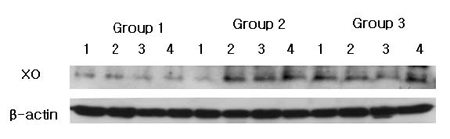
XO western blot analysis showing 4 representative samples in each group. XO levels are quantified on the basis of β-actin levels. XO corresponding to molecular weight 150 kDa is shown to be expressed in all samples including healthy gingiva, and the expression levels of XO are increased in order of group 1, group 2 and group 3. Group 1 : healthy gingiva from systemically healthy person. Group 2 : inflamed gingiva from patient with chronic periodontitis. Group 3 : inflamed gingiva from patient with chronic periodontitis with HTN.
Representative Western blot analysis using MDA specific antibody which detected about 68 kDa molecular weight of MDA were presented in Fig. 3. The levels of normalized MDA expression (ratio of MDA/β-actin) are given in Table 3 and summarized as a graph in Fig. 4. The mean value of MDA expression were 0.741±0.151 in group 1, 0.919±0.116 in group 2 and 1.003±0.081 in group 3. There was a significant difference between group 1 and group 2, between group 1 and group 3. However, there were no significant differences between group 2 and group 3.
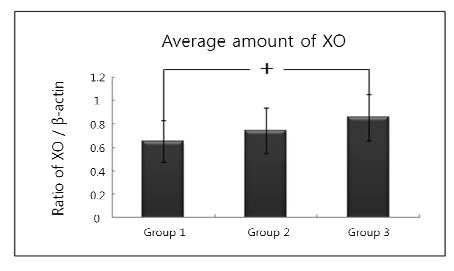
Graphics showing the average amounts (ratio of XO/β-actin) and standard deviation of XO level in groups 1, 2 and 3. In the group 3, the levels of XO are significantly increased as compared to group 1. Group 1 : healthy gingiva from systemically healthy person. Group 2 : inflamed gingiva from patient with chronic periodontitis. Group 3 : inflamed gingiva from patient with chronic periodontitis with HTN.
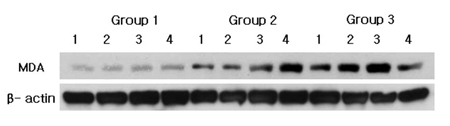
MDA Western blot analysis showing 4 representative samples in each group. MDA levels are quantified on the basis of β-actin levels. MDA corresponding to molecular weight 68 kDa is shown to be expressed in all samples including healthy gingiva, and the expression levels of MDA are increased in order of group 1, group 2 and group 3. Group 1 : healthy gingiva from systemically healthy person. Group 2 : inflamed gingiva from patient with chronic periodontitis. Group 3 : inflamed gingiva from patient with chronic periodontitis with HTN.
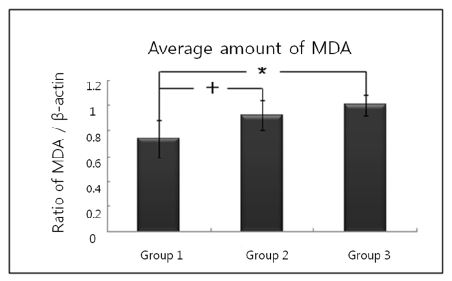
Graphics showing the average amounts (ratio of MDA/β-actin) and standard deviation of MDA level in groups 1, 2 and 3. In the group 2 and group 3, the levels of MDA are significantly increased as compared to group 1. Group 1 : healthy gingiva from systemically healthy person. Group 2 : inflamed gingiva from patient with chronic periodontitis. Group 3 : inflamed gingiva from patient with chronic periodontitis with HTN.
DISCUSSION
It is widely accepted that, toxic effects by increased ROS would cause oxidative damage. Through their cell-damaging effects, ROS have been implicated to play a role in vascular injury associated with CVD, such as HTN, atherosclerosis, restenosis and diabetic vascular complications (Griendling et al., 2000; Landmesser & Harrison, 2001). Small arteries undergo structural remodelling due, in large part, to increased cell growth, cell migration, ECM deposition and inflammation (Intengan & Schiffrin, 2001) in HTN. All of these processes are influenced to varying degrees by ROS. It has been also shown that ROS production increases in the activated phagocytic cells in peripheral blood in periodontitis (Gustafsson & Asman, 1996). Chapple et al. (2007) has shown that the antioxidant capacity decreases and certain oxidative stress biomarkers increase in periodontitis.
The importance of the relationships between periodontitis and systematic diseases are also emphasized. HTN is one of the most common disease and the association between periodontitis and HTN has been studied over the past few years. Oxidative stress may act as a potential common link to explain relationships between HTN and periodontitis. Both conditions show increased serum levels of products derived from oxidative damage, with a pro-inflammatory state likely influencing each other bidirectionally.
However, there are only a few studies have been simultaneously analyzed oxidative materials in periodontitis with or without HTN (Kazarina et al., 2010; Bullon et al., 2009). We simultaneously quantified and compared the expression of XO and MDA in periodontitis patients with or without HTN, in order to understand the diagnostic contribution of these oxidative materials to periodontal destruction accompanied with alveolar bone resorption in HTN patients.
The expression levels of XO were increased in order of group 1, group 2 and group 3. The expressions of XO significantly increased in group 3 as compared to group 1 (p<0.05), but there were no significant difference between group 1 and group 2 and between group 2 and group 3 (p>0.05). It is assumed that XO increase in periodontitis with HTN patients of our study may represent possible XO-generated ROS are implicated in both tissue structural damage and cell signaling interference and can cause lipid peroxidation(LPO).
Berry et al. (2004) reported that XO-generated ROS are implicated in both tissue structural damage and cell signaling interference. In addition to tissue damage, XO-generated ROS can also exert cardiotoxicity via interference with cell signaling (Hellsten-Westing Y, 1993).
ROS-related tissue destruction can be measured by the final product of LPO, such as MDA, which is the principal and most studied product of polyunsaturated fatty acid peroxidation. In this study, the quantitative analysis of MDA level showed that MDA expression was significantly higher in group 2 than in group 1 and the difference was statistically significant (p<0.05). In addition, the quantitative analysis of MDA level also showed that MDA expression was significantly higher in group 3 than group 1 and the difference was statistically significant (p<0.05). But although the MDA level increased in group 3 compared with group 2, there was no significant difference between groups (p>0.05). Gutteridge et al. (1995) has shown that measuring the concentration of LPO products such as MDA could assess the extent of tissue damage. Elevated MDA levels of patients with periodontitis in several studies have been reported. Marton et al. (1993) showed that the MDA content of chronic apical periodontitis tissues was higher than in healthy tissue of the same individuals. Akalin et al. (2007) has shown significantly high levels of MDA in the saliva of patients with periodontitis in comparison with healthy control subjects. Khalili et al. (2008) reported a increase in the salivary MDA activity with an increase in probing depth. These results were agreement with their reports and indicate that an elevated MDA level is markedly related to the clinical status of patients. It is possible, as suggested by Sheikhi et al. (2001), that the increased MDA level could occur through the mechanism of superoxide anion production during the interaction of periodontopatho genes or their products and neutrophils within periodontal tissues or pockets.
It is assumed that MDA increase in periodontitis with HTN patients may be due to excess of oxidative stress because LPO can result in oxidative stress.
In the present study, the difference between group 2 and group 3 of XO and MDA was not statistically significant. However, the expression level of XO and MDA in inflammed gingiva with HTN showed increasing tendency compared to non-hypertensive inflamed gingiva.
Therefore, it is suggested that HTN may induce elevation of oxidation process and consequently influence the changes of antioxidant levels. Pierdomenico et al. (1998) and Lerman et al. (2001) investigated oxidation and peroxidation in hypertensive patients and indicated that HTN caused increased oxidation processes, which emphasized on importance and usefulness of antioxidants.
Reports about the ROS in periodontitis were still controversal, now and the analysis of ROS must accompany the careful consideration of many factors that influence the results of oxidative stress, for instance, the limitation of sample number, the difference of individual sample, the inherent basal antioxidant status, the investigated tissue type (gingival crevicular fluid or gingival tissue), investigation methodology and the correlation among oxidative materials.
In conclusion, it is suggested that oxidative stress in defense system like higher XO and MDA levels seems to reflect increased oxygen radical activity (oxidative stress) during periodontitis and hypertension. It could also be suggested that periodontitis with hypertension might induce elevation of oxidation process and consequently influence the changes of antioxidant levels. But it is currently uncertain whether oxidative stress is a cause or a result of inflammation. Although there are controversal studies and further investigations are needed, these results showed an important correlation between oxidative stress and periodontitis with HTN.
Further investigations are needed to clarify the role of oxidative stress in destructive periodontal disease and we hope that could identify specific therapeutic targets for host-modulating therapies using antioxidants in the future.
SUMMARY
The purpose of this study was to quantify the expressions of XO and MDA in the gingival tissues of the chronic periodontitis patients associated with HTN.
Gingival tissue samples were obtained during periodontal surgery or tooth extraction. Depending on the patient's systemic condition and clinical criteria of gingiva, each gingival sample was divided into three groups. Group 1 (n=16) is clinically healthy gingiva without bleeding and no evidence of bone resorption or periodontal pockets, obtained from systemically healthy 12 patients. Group 2 (n=16) is inflamed gingiva from patients with chronic periodontitis. Group 3 (n=16) is inflamed gingiva from patients with chronic periodontitis associated with HTN. Tissue samples were prepared and analyzed by Western blotting. The relative quantifications of XO and MDA were performed with a densitometer and the results were statistically analyzed by one-way ANOVA followed by Tukey test.
1. The expression levels of XO increased in order of group 1, group 2 and group 3, and significantly increased in group 3 as compared to group 1. But there were no significant difference between group 1 and group 2 and between group 2 and group 3.
2. The expression levels of MDA increased in order of group 1, group 2 and group 3. MDA expression was significantly higher in group 2 than in group 1, and the quantitative analysis of MDA level was significantly higher in group 3 than in group 1. But although the MDA level increased in group 3 compared with group 2, there was no significant difference between groups.
In conclusion, this study demonstrated that the expression levels of XO and MDA might be inflammatory and bone resorption marker in periodontal inflammed tissue. It is assumed that hypertension may be associated with the progression of periodontal inflammation and alveolar bone resorption.
References
- FA Akalin, E Baltacioglu, A Alver, E Karabulut, Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis, J Clin Periodontol, (2007, Jul), 34(7), p558-65.
-
T Ardan, J Kovaceva, J Cejková, Comparative histochemical and immunohistochemical study on xanthine oxidoreductase/xanthine oxidase in mammalian corneal epithelium, Acta Histochem, (2004, Feb), 106(1), p69-75.
[https://doi.org/10.1016/j.acthis.2003.08.001]

-
CE Berry, JM Hare, Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications, J Physiol, (2004, Mar, 16), 555(Pt 3), p589-606.
[https://doi.org/10.1113/jphysiol.2003.055913]

-
AM Briones, RM Touyz, Oxidative stress and hypertension: current concepts, Curr Hypertens Rep, (2010, Apr), 12(2), p135-42.
[https://doi.org/10.1007/s11906-010-0100-z]

-
P Bullon, JM Morillo, MC Ramirez-Tortosa, JL Quiles, HN Newman, M Battino, Metabolic syndrome and periodontitis: is oxidative stress a common link?, J Dent Res, (2009, Jun), 88(6), p503-18.
[https://doi.org/10.1177/0022034509337479]

-
IL Chapple, GR Brock, MR Milward, N Ling, JB Matthews, Compromised GCF total antioxidant capacity in periodontitis: cause or effect?, J Clin Periodontol, (2007, Feb), 34(2), p103-10.
[https://doi.org/10.1111/j.1600-051X.2006.01029.x]

- IL Chapple, JB Matthews, The role of reactive oxygen and antioxidant species in periodontal tissue destruction, Periodontol 2000, (2007), 43, p160-232.
-
JY Cho, S Xing, X Liu, TL Buckwalter, L Hwa, TJ Sferra, , Expression and activity of human Na+/I- symporter in human glioma cells by adenovirus-mediated gene delivery, Gene Ther, (2000, May), 7(9), p740-9.
[https://doi.org/10.1038/sj.gt.3301170]

-
JT Curnutte, BM Babior, Chronic granulomatous disease, Adv Hum Genet, (1987), 16, p229-97.
[https://doi.org/10.1007/978-1-4757-0620-8_4]

-
N Dierckx, G Horvath, C van Gils, J Vertommen, J van de Vliet, I De Leeuw, , Oxidative stress status in patients with diabetes mellitus: relationship to diet, Eur J Clin Nutr, (2003, Aug), 57(8), p999-1008.
[https://doi.org/10.1038/sj.ejcn.1601635]

-
D Ekuni, T Tomofuji, N Tamaki, T Sanbe, T Azuma, R Yamanaka, , Mechanical stimulation of gingiva reduces plasma 8-OHdG level in rat periodontitis, Arch Oral Biol, (2008, Apr), 53(4), p324-9.
[https://doi.org/10.1016/j.archoralbio.2007.10.005]

- Angeli Fabio, Verdecchia Paolo, Pelligrino Concetta, Association between periodontal diseases and left ventricle mass in essential hypertension, Hypertension, (2003, Mar), 41(3), p488-92.
- A Gönenç, Y Ozkan, M Torun, B Simşek, Plasma malondialdehyde (MDA) levels in breast and lung cancer patients, J Clin Pharm Ther, (2001, Apr), 26(2), p141-4.
-
KK Griendling, D Sorescu, B Lassegue, M Ushio-Fukai, Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology, Arterioscler Thromb Vasc Biol, (2000, Oct), 20(10), p2175-83.
[https://doi.org/10.1161/01.ATV.20.10.2175]

- A Gustafsson, B Asman, Increased release of free oxygen radicals from peripheral neutrophils in adult periodontitis after Fc delta-receptor stimulation, J Clin Periodontol, (1996, Jan), 23(1), p38-44.
- JM Gutteridge, Lipid peroxidation and antioxidants as biomarkers of tissue damage, Clin Chem, (1995, Dec), 41(12 Pt 2), p1819-28.
- B Halliwell, Mechanisms involved in the generation of the free radicals, Pathol Biol(Paris), (1996, Jan), 44(1), p6-13.
-
B Halliwell, Oral inflammation and reactive species: a missed opportunity?, Oral Dis, (2000, May), 6(3), p136-7.
[https://doi.org/10.1111/j.1601-0825.2000.tb00324.x]

-
R Harrison, Structure and function of xanthine oxidoreductase: where are we now?, Free Radic Biol Med, (2002, Sep), 33(6), p774-97.
[https://doi.org/10.1016/S0891-5849(02)00956-5]

-
Y Hellsten-Westing, Immunohistochemical localization of xanthine oxidase in human cardiac and skeletal muscle, Histochemistry, (1993, Sep), 100(3), p215-22.
[https://doi.org/10.1007/BF00269094]

-
HD Intengan, EL Schiffrin, Vascular remodeling in hypertension: Roles of apoptosis, inflammation and fibrosis, Hypertension, (2001), 38, p581-7.
[https://doi.org/10.1161/hy09t1.096249]

- LN Kazarina, LV Vdovina, EN Dubrovskaia, Mexidol preparation influence upon lipids peroxide oxidation and oral fluid antioxidant system activity in patients with chronic generalized parodontitis and arterial hypertension, Stomatologiia (Mosk), (2010), 89(2), p18-21.
-
JP Kehrer, The Haber-Weiss reaction and mechanisms of toxicity, Toxicology, (2000, Aug, 14), 149(1), p43-50.
[https://doi.org/10.1016/S0300-483X(00)00231-6]

-
J Khalili, HF Biloklytska, Salivary malondialdehyde levels in clinically healthy and periodontal diseased individuals, Oral Dis, (2008, Nov), 14(8), p754-60.
[https://doi.org/10.1111/j.1601-0825.2008.01464.x]

-
JB Kim, MH Jung, JY Cho, JW Park, JY Suh, JM Lee, The influence of type 2 diabetes mellitus on the expression of inflammatory mediators and tissue inhibitor of metalloproteinases-2 in human chronic periodontitis, J Periodontal Implant Sci, (2011, Jun), 41(3), p109-16.
[https://doi.org/10.5051/jpis.2011.41.3.109]

- JS Kim, DW Jones, SJ Kim, YP Hong, Hypertension in Korea: a national survey, Am J Prev Med, (1994, Jul-Aug), 10(4), p200-4.
-
U Landmesser, DG Harrison, Oxidative stress and vascular damage in hypertension, Coron Artery Dis, (2001, Sep), 12(6), p455-61.
[https://doi.org/10.1097/00019501-200109000-00004]

- JM Lee, TA Libermann, JY Cho, The synergistic regulatory effect of Runx2 and MEF transcription factors on osteoblast differentiation markers, J Periodontal Implant Sci, (2010, Feb), 40(1), p39-44.
-
LO Lerman, KA Nath, M Rodriguez-Porcel, JD Krier, RS Schwartz, C Napoli, , Increased oxidative stress in experimental renovascular hypertension, Hypertension, (2001, Feb), 37(2 Part 2), p541-6.
[https://doi.org/10.1161/01.HYP.37.2.541]

- IJ Marton, G Balla, C Hegedus, P Redi, Z Szilagyi, L Karmazsin, , The role of reactive oxygen intermediates in the pathogenesis of chronic apical periodontitis, Oral Microbiol Immunol, (1993, Aug), 8(4), p254-7.
-
JM Matés, C Pérez-Gómez, I Núñez de Castro, Antioxidant enzymes and human diseases, Clin Biochem, (1999, Nov), 32(8), p595-603.
[https://doi.org/10.1016/S0009-9120(99)00075-2]

- HR Mühlemann, S Son, Gingival sulcus bleeding-a leading symptom in initial gingivitis, Helv Odontol Acta, (1971, Oct), 15(2), p107-13.
-
A Ornoy, Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy, Reprod Toxicol, (2007, Jul), 24(1), p31-41.
[https://doi.org/10.1016/j.reprotox.2007.04.004]

-
RC Page, KS Kornman, The pathogenesis of human periodontitis : an introduction, Periodontol 2000, (1997, Jun), 14, p9-11.
[https://doi.org/10.1111/j.1600-0757.1997.tb00189.x]

- MI Perlstein, NF Bissada, Influence of obesity and hypertension on the severity of periodontitis in rats, Oral Surg Oral Med Oral Pathol, (1997, May), 43(5), p707-19.
-
SD Pierdomenico, F Costantini, A Bucci, D De Cesare, F Cuccurullo, A Mezzetti, Low-density lipoprotein oxidation and vitamins E and C in sustained and white-coat hypertension, Hypertension, (1998, Feb), 31(2), p621-6.
[https://doi.org/10.1161/01.HYP.31.2.621]

-
MC Polidori, K Savino, G Alunni, M Freddio, U Senin, H Sies, , Plasma lipophilic antioxidants and malondialdehyde in congestive heart failure patients: relationship to disease severity, Free Radic Biol Med, (2002, Jan, 15), 32(2), p148-52.
[https://doi.org/10.1016/S0891-5849(01)00782-1]

- C Richter, Biophysical consequences of lipid peroxidation in membranes, Chem Phys Lipids, (1987), 44(2-4), p175-89.
-
DV Sculley, SC Langley-Evans, Salivary antioxidants and periodontal disease status, Proc Nutr Soc, (2002), 61(1), p137-43.
[https://doi.org/10.1079/PNS2001141]

-
M Sheikhi, PK Bouhafs, KJ Hammarström, C Jarstrand, Lipid peroxidation caused by oxygen radicals from Fusobacterium-stimulated neutrophils as a possible model for the emergence of periodontitis, Oral Dis, (2001), 7, p41-6.
[https://doi.org/10.1034/j.1601-0825.2001.70109.x]

- IV Turko, F Murad, Protein nitration in cardiovascular diseases, Pharmacol Rev, (2002, Dec), 54(4), p619-34.
- RJ Waddington, R Moseley, G Embery, Reactive oxygen species : a potential role in the pathogenesis of periodental diseases, Oral Dis, (2000, May), 6(3), p138-51.
- SJ Weiss, Oxygen, ischemia and inflammation, Acta Physiol Scand Suppl, (1986), 548, p9-37.