Evaluation of component release and oxygen-inhibited layer removal on cytotoxicity of syringe-type bis-acryl composites for provisional restorations
Abstract
This study aimed to analyze the eluted components of syringe-type bis-acryl composites and to evaluate the effect of removing the oxygen-inhibited layer on cytotoxicity. Four different bis-acryl provisional composite materials-Protemp 4 (PT), Structur 2 SC (ST), Luxatemp Automix Plus (LT), and Hexa-Temp (HT)-were evaluated. Gas chromatography/mass spectrometry was used to analyze the composite eluate after 24 h of immersion in methanol. An agar overlay test and a live/dead assay were performed on the polymerized disc-shaped specimens after 24 h. To evaluate the effect of removing the oxygen-inhibited layer, samples were prepared with a surface oxygen-inhibited layer. The surface oxygen-inhibited layer of the disc-shaped specimens was removed with alcohol only (subgroup A) or with polishing and alcohol (subgroup PA), and their cytotoxicities were compared with those of “as received” (subgroup N) specimens using the WST-1 assay. Statistical significance was assessed using analyses of variance, followed by Bonferroni multiple comparison tests (α=0.05). Different components were detected by gas chromatography/mass spectrometry analysis among the groups. The agar overlay assay confirmed severe cytotoxicity in HT, LT, and ST groups, whereas PT showed moderate cytotoxicity. The effect of removing the oxygen-inhibited layer on cell viability was significantly higher in PA than in N in all composite groups. In HT and ST, the cell viability was significantly higher in PA than in A. Syringe-type bis-acryl composites for provisional restorations may elute various components into the oral cavity, which may cause cytotoxicity in adjacent structures. The cytotoxicity of the materials is reduced by the removal of the oxygen-inhibited layer.
초록
본 연구의 목적은 시린지 형태의 비스아크릴 기반 임시치관용 자가중합형 복합레진의 용출물을 분석하고, 산소중합억제층 제거 방법이 세포독성에 미치는 영향을 평가하고자 하였다. 평가를 위해 시판 중인 네 가지 임시치관용 비스아크릴 복합레진을 비교 분석하였다. 실험에는Protemp 4(PT), Structur 2 SC(ST), Luxatemp Automix Plus(LT)과 Hexa-Temp(HT)를 사용하였다. 원반형 레진 시편을 제작하여 메탄올에서 24시간 용출 후, 기체 크로마토그래피/질량 분석법(GC/MS)을 사용하여 용출물을 분석하였다. 동일하게 제작된 원반형 시편으로 세포독성을 평가하기 위해 한천중층시험법과 live/dead assay를 사용하였다. 산소중합억제층 제거 영향을 평가하기 위해 산소중합억제층을 재현한 원반형 시편을 제작하였다. 표면 처리 방법에 따라 세 개의 하위집단인, 처리하지 않은 대조군(N), 산소중합억제층을 알코올로 문질러 제거한 군(A), 표면 연마 후 알코올로 문질러 제거한 군(PA)으로 나누었다. 세포독성은 시편을 배지에 용출하여WST-1으로 평가하였다. 통계분석을 위해 소프트웨어를 사용하였으며, 등분산과 정규성 검정 후, ANOVA와 Bonferroni 사후분석법으로 통계적 유의성을 평가하였다(α=0.05). GC/MS 분석 결과, 실험군에 따라 서로 다른 구성성분이 용출되었다. 한천중층시험법 결과, 세포독성은 HT, LT, ST에서 심한독성, PT에서는 중등도의 독성이 나타났다. 산소중합억제층 제거에 의한 세포생존은 모든 레진에서 N에 비해 PA 가 유의하게 높았으며, HT와 ST는 A에 비해 PA에서 유의하게 높았다. 시린지 형태의 임시치관용 자가중합형 복합레진은 구성성분에 따라 구강 내 세포독성에 영향을 줄 수 있는 다양한 용출물이 구강 내로 용출될 수 있으며, 임시치관 재료의 독성은 산소중합억제층 제거하는 방법에 따라 감소할 수 있다.
Keywords:
Bis-acryl composite, Gas chromatography/mass spectrometry, Oxygen-inhibited Layer, Cytotoxicity키워드:
자가중합형 비스아크릴 복합레진, 용출, 산소중합억제층, 세포독성Introduction
For successful permanent restorations, properly fabricated provisional restorations are crucial. The provisional restoration should remain satisfactory for chewing, occlusion, pronunciation, and esthetics for at least a few days to several months before the final restoration is delivered (1, 2).
Acrylic resin is the most popular conventional provisional material and is mostly used in the form of a powder mixed with liquid. The main components are polymethyl methacrylate (PMMA) and methyl methacrylate (MMA) (2, 3). The limitations of this material include void entrapment, characteristic heat generation, and shrinkage that occur during the polymerization process, which might affect the pulp integrity and quality of the provisional restorations (3, 4).
A bis-acryl composite is another type of provisional material with less heat generation and shrinkage during polymerization (5, 6). It is used with auto-mix dispensers, making it convenient with fewer voids (7). Bis-acryl composites are considered more accurate and durable, particularly when the span of the restoration increases (8, 9).
As the provisional material remains in direct contact with intraoral structures, concerns regarding the leaching of residual components into the oral cavity are frequently raised. Bisphenol A-glycidyl methacrylate (Bis-GMA), urethane dimethacrylate (UDMA), triethylene glycol dimethacrylate (TEGDMA), and bisphenol A (BPA) reportedly cause DNA double-strand breakage, increased inflammatory cytokine and enzyme activities, and potential acute systemic toxicity (10-12). Therefore, the provisional material should not be cytotoxic or affect the integrity of the dentin-pulp complex and periodontal tissues.
To reduce the possible cytotoxicity, the removal of the remaining unpolymerized material at the surface is recommended (13, 14). An oxygen-inhibited layer is known to reduce the degree of monomer conversion and cell viability (15). However, studies on the cytotoxicity of bis-acryl composites after using different removal methods for the oxygen-inhibited layer are lacking.
This study aimed to analyze the eluted components of syringe-type bis-acryl composites and to evaluate the effect of oxygen-inhibited layer removal on cytotoxicity so as to gain insights into the relationship between eluted monomers and potential cytotoxicity. The null hypothesis is as follows: there are no differences in component elution among the composites, in cytotoxicity among the composites, and in cytotoxicity among the oxygen-inhibited layer removal methods.
Materials and Methods
1. Composite sample preparation
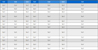
Four different bis-acryl provisional composite materials-Protemp 4 (PT), Structur 2 SC (ST), Luxatemp Automix Plus (LT), and Hexa-Temp (HT)-were used in this study (Table 1).
For the eluted component analysis, agar overlay assay, and live/dead Assay, the composites were filled in a Teflon® mold (10 mm in diameter and 2 mm in height), and the surface was covered with a Mylar® strip to prevent the formation of the inhibition layer. After 10 min of setting time, the specimens were removed from the mold and used for the experiments, following the manufacturers’ instructions.
For evaluating the effect of removal of the oxygen-inhibited layer, the composites were filled in a Teflon® mold (10 mm in diameter and 2 mm in height), and the surface remained uncovered for the oxygen-inhibited layer to form. The specimens were divided into three N, A, and PA subgroups according to the surface-finishing method. The specimens of subgroup N (as-received) were compared with those of subgroup A (the surface layer was wiped off with an alcohol sponge) and subgroup PA (the surface was polished and wiped with an alcohol sponge). For subgroup A, the surface was wiped with a 70% ethyl alcohol-dampened sponge (Meditop, Seoul, Korea) until the surface gloss disappeared. For subgroup PA, the surface was serially polished using silicon carbide abrasive papers with grit sizes of 320, 600, 800, 1200, and 2,400 (MECATECH 250 SPC; Perci, Grenoble, France), and the surface was subsequently wiped with a 70% ethyl alcohol-dampened sponge (Meditop).
2. Gas chromatography/mass spectrometry
The disc-shaped specimens were immersed in 99.9% methanol (Sigma-Aldrich, St. Louis, MO, USA) and eluted for 24 h at 37 ℃ in a brown glass vial (3cm2/mL). To qualitatively evaluate the eluates, a Trace Ultra GC Ultra gas chromatograph (GC) linked to a triple quadrupole mass spectrometer (MS; TSQ 8000; Thermo Fisher Scientific, Madison, WI, USA) was used in the splitless mode. The compounds were separated using a GC column, with geometry parameters of 60 m length, 0.25 mm diameter, and 0.25 μm film thickness, at a stationary phase with a split ratio of 1:10 and helium flowing at a constant rate of 1 mL/min. The GC oven was heated isothermally at 50 ℃ for 2 min, heated to 280 ℃ (25 ℃/min), which was maintained for 5 min, and then cooled to 250 ℃. With an electron ionization source temperature of 240 ℃, the MS was set to the full scan mode, and data were recorded (mass-to-charge ratio, m/z 50-600) at 70 eV. For qualitative analysis, the relevant compounds were identified by comparing their retention times and mass spectra with their corresponding reference standards and the National Institute of Standards and Technology (NIST) library database.
3. Cell culture
A human gingival fibroblast cell line (HGF-1; ATCC CRL-2014) was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, Logan, UT, USA) containing 1% penicillin (Gibco, Life Technologies, Grand Island, NY, USA), 1% streptomycin (Gibco), and 10% fetal bovine serum (FBS; Gibco), at 37 ℃ in a humidified chamber with 5% CO2.
4. Cytotoxicity evaluation
For the agar overlay assay, HGF-1 cells were seeded in 100 mm-diameter cell culture dishes and incubated to the confluency of 80% at 37 ℃ in 5% CO2. The medium was replaced with 10 mL of freshly prepared agar medium containing DMEM, 10% FBS, and 1.5% agarose mixture. A total of 10 mL of 0.02% neutral red solution (Sigma) in phosphate-buffered saline (PBS; Lonza, Morristown, NJ, USA) was added, and the cells were incubated for 20 min at 37 ℃. Excess dye was then removed, and the PT, ST, HT, and LT specimens were placed on the agar surface, along with a negative control (NC) and positive control (PC). A latex glove was used as the PC, and a cover glass was used as the NC. The dishes were incubated for 24 h at 37 ℃ in 5% CO2 and examined under a stereomicroscope (KI-2000F; Korea Lab Tech, Seong Nam, Gyeonggido, Korea). The decolorized zones and cell lysis were evaluated according to the International Organization for Standardization (ISO) 7405:2018 (Tables 2 and 3) (13).
For the live/dead assay, HGF-1 cells were seeded in a 35-mm confocal dish (SPL) at a concentration of 2×104 cells/well and incubated for 24 h. The cells were then treated with 1 mL of eluates prepared in DMEM for 24 h at 37 ℃. After incubation, the cells were stained using the LIVE/DEADTM Viability kit (Invitrogen, Waltham, MA, USA) for 30 min and observed under a digital inverted fluorescence microscope (DS-Ri2; Nikon, Tokyo, Japan). Live cells emitted green fluorescence, whereas dead cells emitted red fluorescence.
5. WST-1 assay for N, A, and P subgroups
For Water-Soluble Tetrazolium-1 (WST-1) assay, the specimens of N, A, and PA were placed in a 24-well plate (SPL) with 1 mL of DMEM and stored at 37 ℃. After 24 h of storage, the HGF-1 cells were seeded on the composite surface at a concentration of 2×104 cells/mL and incubated for 24 h. The EZ-Cytox Cell Viability Assay Kit (DoGenBio, Seoul, Korea) was used according to the manufacturer’s instructions. Optical density (OD) was measured at 450 nm using a microplate reader (Allsheng AMR-100; Hangzhou, Zhejiang, China). The cell viability percentage is expressed as the ratio of the OD of the experimental groups to that of the cell culture dish.
6. Statistical analysis
After the normality test and equal variance test of each data point, statistical analysis was performed with an analysis of variance followed by a Bonferroni multiple comparison test. Data were analyzed at a significance level of 0.05 using GraphPad Prism 9.0.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
1. GC/MS analysis of composite eluates
Representative GC/MS chromatograms of the substances from each composite eluate are depicted in Figure 1 and Table 4. Methylbenzene, butylated hydroxytoluene (BHT), and N,N-dibutylphenethylamine (N, N-DMPEA) were the three main components of PT. p-Cymene, benzoic acid (BA), tributyl acetyl citrate (ATBC), and p-Tolyldiethanolamine (DEPT) were detected in ST. TEGDMA, DEPT, Tinuvin P (TINP), and GDMA were the main components detected in HT. Hydroxyethyl methacrylate (HEMA), TEGDMA, camphorquinone (CQ), and 2-hydroxy-4-methacryloxybenzophenone were mainly detected in LT.
2. Cytotoxicity evaluation with agar overlay and live/dead assays
In the agar overlay assay, all the groups showed decolorization of the composite specimens (Table 5). PT scored a lower lysis index than HT, LT, and ST. Severe cytotoxicity was confirmed in ST, LT, and HT, whereas PT showed moderate cytotoxicity.
The live/dead staining assay results, after 24 h of composite extract treatment, are presented in Figure 2. PT, LT, and HT showed a higher density of live cells than ST.
3. Cell viability of N, A, and P subgroups
The cells directly seeded on the materials showed a more significant decrease in cell viability than those seeded on the culture dish (Figure 3). A significant difference was observed between N and PA in all the groups. PT-PA (45.05±9.29) and ST-PA (38.25±8.03) showed a significantly higher cell viability than PT-N (34.74±3.73, p=0.0131) and ST-N (26.08±2.45, p=0.0025), respectively. Similarly, HT-PA (51.87±14.34) and LT-PA (26.31±4.77) showed a significantly higher cell viability than HT-N (37.73±4.49, p=0.0004) and LT-N (15.93±1.42, p=0.0121), respectively. In ST and HT, subgroup PA increased significantly more than subgroup A (p=0.0048 and p=0.0071, respectively).
Discussion
Based on the GC/MS analysis results, the main components that were detected were different in the four bis-acryl composites. The agar overlay assay showed moderate-to-severe cytotoxicity. After removal of the oxygen-inhibited layer, the cell viability increased, especially in the polished and alcohol-combined group. Therefore, the three null hypotheses are rejected.
In terms of the eluted component analysis, this study focused on the components with low molecular weight (m/z 35–800), which are more readily released in the oral cavity than high-molecular-weight monomers, contributing to the structural stability of the polymerized composites. Among the major components of dental composites, the monomers contribute to fibroblast cytotoxicity in the order of Bis-GMA, UDMA, TEGDMA, and HEMA (16, 17). The GC/MS analysis used in this study was able to detect HEMA, TEGDMA, and BPA.
For the monomers, no trace of UDMA was detected in PT, similar to the result of a previous HPLC analysis (18). Meanwhile, HEMA, a product of the reaction of methanol and UDMA during high-temperature (280 ℃) vaporization in the GC process, was detected in HT and LT (19). HEMA is a monomer known to induce cytotoxicity by inducing oxygen stress, genotoxicity, and apoptosis (20). Because HEMA was not listed as a main component of HT and LT, we assume that HEMA is a breakdown product of UDMA (Table 1). TEGDMA was detected in HT and LT, and this low molecular weight co-monomer is reported to induce dose-dependent and time-related apoptosis (21). We assume that this unpolymerized monomer was released, resulting in increased cytotoxicity. BPA, a trace component of Bis-GMA, was not detected in any of the tested composites.
Among the mainly detected additive elements, BA and methyl benzoate (MeBA), which are the decomposition products of benzoyl peroxide (BPO) in methanol, were detected in ST and HT (22). BPO was used as the initiator in both ST and HT (Table 1). BPO is known to have a genotoxic effect that contributes to cytotoxicity (23, 24). In ST, in addition to other components, DEPT and ATBC were detected. ATBC is a phthalate substitute used to increase the flexibility and durability of materials. It is generally considered safe; however, concerns regarding ovarian toxicity at low doses exist (25). In LT, a photosensitizer CQ was detected, CQ is reported to increase produce reactive oxygen species when leached (26).
The surface-finishing method of bis-acryl composites varies. As saving time is crucial in clinical situations, ethanol is recommended to wipe-off the polymerized surface so as to minimize the need for an additional working step in some products (27). In the alcohol-wiped-off group (subgroup A), the cell viability was not affected compared with the untreated group (subgroup N). Based on a previous study, the oxygen-inhibiting layer may have remained on the composite surface even after removal with ethanol (28). In subgroup N, specimens with an inhibition layer remained on the upper surface, and the cell viability was below 70%, which is generally recommended by ISO 10993-5:2009. This reduced cell viability is due to an exaggerated level of component release from the unpolymerized surface (29). The considerable removal of the oxygen-inhibited layer positively affects cell viability, as in other composite materials, by reducing possible eluates (15, 30, 31).
Regarding the multiple factors influencing the release and toxicity of components, the determination of the exact amount of toxic components in a clinical situation is difficult. The magnitude of the released component will differ according to the solvent used. Furthermore, under physiological conditions, different components can be released by protein binding, and dilution or decomposition of the components owing to salivary function is inevitable (32). In future studies, extended chemical analysis is recommended to evaluate the main components in relation to the surface oxygen inhibition layer. Additionally, possibilities remain for the elution of high molecular weight monomers, as since the degree of conversion of the bis-acryl composites ranged from 40–60% (33, 34).
Conclusions
Syringe-type bis-acryl composites for provisional restorations may elute various components into the oral cavity, which may cause cytotoxicity in adjacent structures. The removal of the oxygen-inhibited layer by a polishing combined with an alcohol sponge wipe-off method reduced the cytotoxicity of the bis-acryl composite materials.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A2C1102316)
References
-
Miura S, Fujisawa M, Komine F, Maseki T, Ogawa T, Takebe J, et al. Importance of interim restorations in the molar region. J Oral Sci. 2019; 61(2):195-9.
[https://doi.org/10.2334/josnusd.19-0102]

-
Gratton DG, Aquilino SA. Interim restorations. Dent Clin North Am. 2004;48(2):487-97.
[https://doi.org/10.1016/j.cden.2003.12.007]

-
Zafar MS. Prosthodontic applications of polymethyl methacrylate (PMMA): An update. Polymers. 2020;12 (10):2299.
[https://doi.org/10.3390/polym12102299]

- Athar Z, Juszczyk AS, Radford DR, Clark RK. Effect of curing cycles on the mechanical properties of heat cured acrylic resins. Eur J Prosthodont Restor Dent. 2009;17(2):58-60.
-
Leprince JG, Palin WM, Hadis MA, Devaux J, Leloup G. Progress in dimethacrylate-based dental composite technology and curing efficiency. Dent Mater. 2013;29(2):139-56.
[https://doi.org/10.1016/j.dental.2012.11.005]

-
Kim SH, Watts DC. Polymerization shrinkage-strain kinetics of temporary crown and bridge materials. Dent Mater. 2004;20(1):88-95.
[https://doi.org/10.1016/S0109-5641(03)00101-5]

-
Pituru SM, Greabu M, Totan A, Imre M, Pantea M, Spinu T, et al. A review on the biocompatibility of PMMA-based dental materials for interim prosthetic restorations with a glimpse into their modern manufacturing techniques. Materials. 2020;13(13):2894.
[https://doi.org/10.3390/ma13132894]

-
Schwantz JK, Oliveira-Ogliari A, Meereis CT, Leal FB, Ogliari FA, Moraes RR. Characterization of bis-acryl composite resins for provisional restorations. Braz Dent J. 2017;28(3):354-61.
[https://doi.org/10.1590/0103-6440201601418]

-
Madhavan S, Jude SM, Achammada S, Ullattuthodi S, Kuriachan T, Jacob J. Comparison of marginal accuracy of interim restoration fabricated from self-cure composite and autopolymerizing acrylic resin: An in vitro study. J Pharm Bioallied Sci. 2020;12(Suppl 1):S361-6.
[https://doi.org/10.4103/jpbs.JPBS_106_20]

-
Peskersoy C, Oguzhan A, Gurlek O. The effect of flowable composite resins on periodontal health, cytokine levels, and immunoglobulins. BioMed Res Int. 2022;2022:e674597.
[https://doi.org/10.1155/2022/6476597]

-
Lee DH, Kim NR, Lim BS, Lee YK, Yang HC. Effects of TEGDMA and HEMA on the expression of COX-2 and iNOS in cultured murine macrophage cells. Dent Mater. 2009;25(2):240-6.
[https://doi.org/10.1016/j.dental.2008.05.014]

-
Urcan E, Scherthan H, Styllou M, Haertel U, Hickel R, Reichl FX. Induction of DNA double-strand breaks in primary gingival fibroblasts by exposure to dental resin composites. Biomaterials. 2010;31(8); 2010-4.
[https://doi.org/10.1016/j.biomaterials.2009.11.065]

-
Lee MJ, Kim MJ, Kwon JS, Lee SB, Kim KM. Cytotoxicity of light-cured dental materials according to different sample preparation methods. Materials. 2017;10(3):288.
[https://doi.org/10.3390/ma10030288]

-
Yoshii E. Cytotoxic effects of acrylates and methacrylates: relationships of monomer structures and cytotoxicity. J Biomed Mater Res. 1997;37(4):517-24.
[https://doi.org/10.1002/(SICI)1097-4636(19971215)37:4<517::AID-JBM10>3.0.CO;2-5]

-
Jagdish N, Padmanabhan S, Chitharanjan AB, Revathi J, Palani G, Sambasivam M, et al. Cytotoxicity and degree of conversion of orthodontic adhesives. Angle Orthod. 2009;79(6):1133-8.
[https://doi.org/10.2319/080808-418R.1]

-
Martinez-Gonzalez M, Fidalgo-Pereira RC, Torres O, Silva F, Henriques B, Özcan M, et al. Toxicity of resin-matrix cements in contact with fibroblast or mesenchymal cells. Odontology. 2022; Epub ahead of print.
[https://doi.org/10.1007/s10266-022-00758-w]

-
Cebe MA, Cebe F, Cengiz MF, Cetin AR, Arpag OF, Ozturk B. Elution of monomer from different bulk fill dental composite resins. Dent Mater. 2015;31(7):e141-9.
[https://doi.org/10.1016/j.dental.2015.04.008]

-
Hampe T, Wiessner A, Frauendorf H, Alhussein M, Karlovsky P, Bürgers R, et al. A comparative in vitro study on monomer release from bisphenol A-free and conventional temporary crown and bridge materials. Eur J of Oral Sci. 2021;129(6):e12826.
[https://doi.org/10.1111/eos.12826]

-
Michelsen VB, Moe G, Skålevik R, Jensen E, Lygre H. Quantification of organic eluates from polymerized resin-based dental restorative materials by use of GC/MS. J Chromatogr B. 2007;850(1-2):83-91.
[https://doi.org/10.1016/j.jchromb.2006.11.003]

-
Gallorini M, Cataldi A, Di Giacomo V. HEMAi-nduced cytotoxicity: oxidative stress, genotoxicity and apoptosis. Int Endod J. 2014;47(9):813-8.
[https://doi.org/10.1111/iej.12232]

-
Janke V, Von Neuhoff N, Schlegelberger B, Leyhausen G, Geurtsen W. TEGDMA causes apoptosis in primary human gingival fibroblasts. J Dent Res. 2003;82(10):814-8.
[https://doi.org/10.1177/154405910308201010]

-
Hongo T, Hikage S, Sato A. Stability of benzoyl peroxide in methyl alcohol. Dent Mater J. 2006;25(2):298-302.
[https://doi.org/10.4012/dmj.25.298]

-
Babich H, Zuckerbraun H, Wurzburger B, Rubin Y, Borenfreund E, Blau L. Benzoyl peroxide cytotoxicity evaluated in vitro with the human keratinocyte cell line, RHEK-1. Toxicology. 1996;106(1-3):187-96.
[https://doi.org/10.1016/0300-483X(95)03189-M]

-
Yavuz A, Topaktaş M, İstifli ES. In vitro genotoxic 184 effects of benzoyl peroxide in human peripheral lymphocytes. Turk J Biol. 2010;34(1):15-24.
[https://doi.org/10.3906/biy-0808-13]

-
Rasmussen LM, Sen N, Liu X, Craig ZR. Effects of oral exposure to the phthalate substitute acetyl tributyl citrate on female reproduction in mice. J Appl Toxicol. 2017;37(6):668-75.
[https://doi.org/10.1002/jat.3413]

-
Atsumi T, Iwakura I, Fujisawa S, Ueha T. The production of reactive oxygen species by irradiated camphorquinone-related photosensitizers and their effect on cytotoxicity. Arch Oral Biol. 2001 1;46(5):391-401.
[https://doi.org/10.1016/S0003-9969(01)00005-X]

-
Tupinambá ÍVM, Giampá PCC, Rocha IAR, Lima EMCX. Effect of different polishing methods on surface roughness of provisional prosthetic materials. J Indian Prosthodont Soc. 2018;18(2):96-101.
[https://doi.org/10.4103/jips.jips_258_17]

-
Panchal AC, Asthana G. Oxygen inhibition layer: a dilemma to be solved. J Conserv Dent. 2020;23(3):254-8.
[https://doi.org/10.4103/JCD.JCD_325_19]

-
Tang AT, Liu Y, Björkman L, Ekstrand J. In vitro cytotoxicity of orthodontic bonding resins on human oral fibroblasts. Am J Orthod Dentofac Orthop. 1999;116(2):132-8.
[https://doi.org/10.1016/S0889-5406(99)70209-X]

-
Mohsen N, Craig RG, Hanks CT. Cytotoxicity of urethane dimethacrylate composites before and after aging and leaching. J Biomed Mater Res.1998;39(2):252-60.
[https://doi.org/10.1002/(SICI)1097-4636(199802)39:2<252::AID-JBM12>3.0.CO;2-F]

-
Çimen C, Demirsoy FF, Özdemir A, Ark M, Özalp N. Effect of Finishing-Polishing Procedures on Cytotoxicity of Resin-Based Restorative Materials via Real-Time Cell Analysis. J Clin Pediatr Dent. 2022;46(1):24-9.
[https://doi.org/10.17796/1053-4625-46.1.5]

-
Rothmund L, Shehata M, Van Landuyt KL, Schweikl H, Carell T, Geurtsen W, et al. Release and protein binding of components from resin based composites in native saliva and other extraction media. Dent Mater. 2015;31(5):496-504.
[https://doi.org/10.1016/j.dental.2015.01.016]

-
Kim SH, Watts DC. Degree of conversion of bis-acrylic based provisional crown and fixed partial denture materials. J Korean Acad Prosthodont. 2008;46(6):639-43.
[https://doi.org/10.4047/jkap.2008.46.6.639]

-
Padunglappisit C, Posaya-Anuwat S, Sompoch V, Piyawiwattanakoon P, Panpisut P. Effects of different amine activators on the monomer conversion, biaxial flexural strength, and color Stability of experimental provisional dental restorations. Eur J Dent. 2021;15 (03):488-94.
[https://doi.org/10.1055/s-0040-1721908]