
C-reactive Protein Detection in Gingival Crevicular Fluid as an Acute Systemic Inflammation Biomarker in Patients with Severe Periodontitis
C-reactive protein (CRP)은 급성전신감염 시 신체에서 발견되는 생체표지자이다. 최근의 관련 연구에 의하면 급성전신감염의 생체표지자인 CRP가 치주염환자의 혈액에서 발견된다는 보고가 있었고 이러한 높은 급성전신감염상태에서는 치료의 예후가 좋지 않다고 알려져 있다. 이번 연구는 치과에 내원한 환자의 급성전신감염상태를 가늠하기 위한 CRP라는 생체표지자가 심한 치주질환 환자에게서 타액과 치은열구액을 통해 손쉽게 측정가능한지 살펴보는 것이다. 14명의 건강한 사람을 대조군으로 11명의 심한 치주질환를 실험군으로 모집하였다. 타액과 치은열구액을 획득하여 CRP의 농도를 효소결합 면역 분석법을 통하여 측정하였다. 측정결과 치은열구액에서 채취한 CRP의 농도가 심한 치주염환자에서 건강한 사람에 비해 유의차 있게 높았던 반면, 타액에서는 유의차가 나타나지 않았다. 이번 연구를 통해서 치과치료의 예후에 영향을 미치는 CRP를 희석되기 쉬운 타액보다 치은열구액을 통해서 측정하는 것이 바람직하다.
Keywords:
C-reactive protein, gingival crevicular fluid, saliva, severe periodontitisINTRODUCTION
Some research has indicated that inflammation in the target area for dental surgery decreases the success rate of orofacial surgery and oral implant surgery. Inflammation can arise from resident bacteria and other flora or the presence of a foreign body during surgery. (Turvey et al., 2011) To avoid this disadvantage, most surgeons perform blood tests to assess acute systemic inflammation before surgery. This test typically evaluates C-reactive protein (CRP) and interleukin (IL)-6 levels because these are common markers of acute inflammation. Although this information aids in determining the prognosis for recovery, performing the blood test regularly is often untenable because of needle phobia on the part of the patient and turnaround time for results.
For these reasons, some researchers have turned to IL-6 detection in saliva instead of serum due to their easy approach.(Tishler et al., 1999) But evidence has suggested no significant association between IL-6 levels in serum and in saliva under baseline conditions because IL-6 is not only under systemic regulation but also is under local regulation.(Sjogren et al., 2006) Recently, CRP, a common marker of systemic acute inflammation secreted by liver cells under the transcriptional control of IL-6,(Pepys and Hirschfield, 2003) became another target for measurement in saliva for association with serum CRP levels.(Ouellet-Morin et al., 2011) Saliva arises mostly from the salivary gland, but it also contains some gingival crevicular fluid (GCF). Cytokines in GCF are not easily diluted while cytokines in saliva can be diluted by salivary gland stimulation. Therefore, in some studies, GCF instead of saliva has been used for detecting inflammation-related molecules and cytokines.(Dutzan et al., 2009; Shaddox et al., 2011)
Periodontitis is an inflammatory disease well-known in dental departments. Periodontal pathogens affect both local and systemic immune and inflammatory responses, and cytokines from systemic immune and inflammatory responses against periodontitis are involved in destruction of both periodontal connective tissue and alveolar bone.(Noack et al., 2001) One of the cytokines that initiates a systemic acute-phase response is CRP. Recent cross-sectional studies have shown that plasma CRP in periodontitis is elevated compared with controls. (Paraskevas et al., 2008) Therefore, the aims of this study were to confirm the presence of CRP in mouth fluid, which consists of saliva and GCF, in severe chronic periodontitis patients and to assess differences in CRP concentrations in these two fluids and between healthy people and severe chronic periodontitis patients.
MATERIALS AND METHODS
Selection of the test group
Eleven patients with generalized chronic periodontitis, presenting at the Integrated Medical Care Center in Yonsei Dental Hospital, were selected as the test group. The condition of generalized chronic periodontitis was diagnosed by measuring clinical attachment loss (the distance from the cement–enamel junction to the bottom of the pocket) at the Center, using the periodontal probe in six sites of each tooth according to World Health Organization guidelines: mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, and disto-lingual. If teeth had at least one site with > 6 mm clinical attachment loss and the total number of involved teeth was > 9, the patient was diagnosed as having generalized chronic periodontitis. All patients were examined for overall oral condition, gingivitis and periodontitis index, and their premedical histories were checked using a questionnaire (Silness and Löe, 1964). The presence of a minimum of 20 remaining teeth with average gingivitis score over 1.5 was also confirmed. Patients who had histories of cardiovascular disorders (such as coronary artery disease), diabetes mellitus, malignant diseases, immunodeficiency, current pregnancy or lactation, or Alzheimer’s disease were excluded.
Fourteen people were selected from the Yonsei Dental College as the control group. The control group consisted of individuals without a history of periodontal disease and who had been non-smokers throughout their lives. As for the test group, all participants in the control group had at least 20 remaining teeth and were in good general health. Potential control participants were excluded for the same features in their medical histories as for the test group. Members of the control group had no site of clinical attachment loss > 3 mm.
The complete protocol was fully explained to all participants (Table 1), and they agreed with the purpose of this experimental research.
The GCF was collected by insertion of a paper strip into the 1–2 mm of the crevice for 30 seconds (Figure 1a). The site of the GCF collection was selected from among the diseased teeth for the test group and from among healthy teeth for the control group. Each individual tooth was isolated with cotton rolls and the site gently air-dried with an air syringe prior to GCF collection. Following collection, strips were placed in a labelled test tube containing 300 μL of phosphatebuffered saline (PBS) and shaken for 20 minutes. Strips were then removed and the tube centrifuged at 5800 ×g (4500 rpm) to remove plaque and cellular elements. The samples were frozen at −80°C until further analysis. A saliva sample was collected from participants. They were asked to tilt their heads forward, allow the saliva to pool on the floor of the mouth for 1 minute, and then spit the passive drool into a collection container (Figure 1b). The following precautions were taken prior to saliva collections: (1) Each saliva sample was collected at least 60 minutes after consumption of a major meal; (2) each sample was gathered at least 12 hours after alcohol consumption; and (3) participants were asked to rinse their mouths thoroughly with water for 5 minutes. The collected samples were frozen at or below −20°C immediately after the collection to avoid bacterial contamination and any loss of CRP.
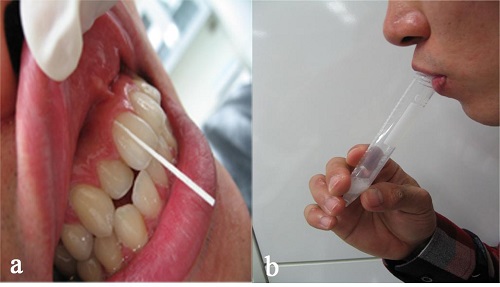
The gingival crevicular fluid (GCF) was collected by insertion of a paper strip into the 1–2 mm of the crevice for 30 seconds (a). A saliva sample was collected by following procedures; tilting their heads forward, allowing the saliva to pool on the floor of the mouth for 1 minute, and then spitting the passive drool into a collection container (b).
Measuring CRP concentration of samples
Measuring CRP concentration of samples with standard CRP conentrations were performed according to the CRP assay kit instructions (1-3302, Salimetric, USA). Briefly, the saliva and the GCF extracts were diluted by mixing 15 μL of each sample with 135 μL of CRP Sample Diluent. A total of 50 μL of each diluted sample was then placed into respective wells of a 96-well plate and 50 μL of CRP Sample Diluent added to two wells to serve as the blank. 150 μL of enzyme-conjugate solution was added to each well using a multichannel pipette. The plate was covered with the adhesive cover provided and incubated at room temperature for 2 hours, under constant mixing at 500 rpm. After washing, 200 μL of TMB solution was added to each well with a multichannel pipette. The plate was incubated in the dark at room temperature for 30 minutes with constant mixing at 500 rpm. Then, 50 μL of stop solution was added with a multichannel pipette and the optical density was read in a microplate reader at 450 nm 10 minutes after the addition of stop solution. The optical density of each sample was measured against the standard to obtain the CRP concentration.
Statistics
Two-sample t-tests were used for analysing differences between groups. The significance level was set at 95%.
RESULTS
Figure 2 shows the optical density values for 3000 pg/mL, 1500 pg/mL, 750 pg/mL, 375 pg/mL, 187.5 pg/mL, and 93.75 pg/mL CRP concentrations and the calibration curve with those six points. Table 2 shows that the R-square value was 0.997 and slope value of the calibration curve was 3.77 × 10–5. Figure 3 illustrates the CRP concentrations in saliva and GCF. In saliva, the healthy group had an average CRP concentration of 891 pg/ml (SD = 2022, n = 14) while chronic periodontitis patients had an average concentration of 3730 pg/ml (SD = 4574, n = 11). The groups did not differ significantly (P > .05). Average CRP concentration in the GCF which was diluted in 300 μL of phosphate-buffered saline (PBS) of healthy controls was calculated at 118 pg/ml (SD = 179); that of chronic periodontitis patients was 6226 pg/ml (SD = 5867). Twosample t-tests confirmed a statistical difference between healthy people and chronic periodontitis patients for CRP concentration in GCF (P < .05).
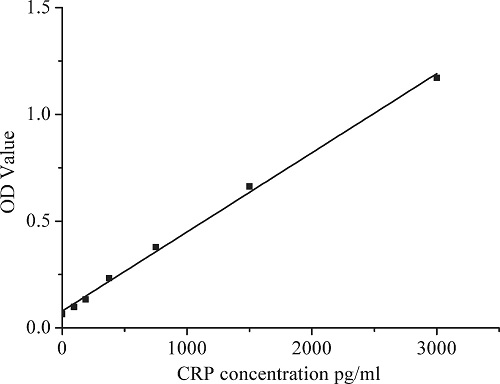
Calibration curve with six optical density of prepared C-reactive protein concentration: 3000 pg/mL, 1500 pg/mL, 750 pg/mL, 375 pg/mL, 187.5 pg/mL, and 93.75 pg/m.
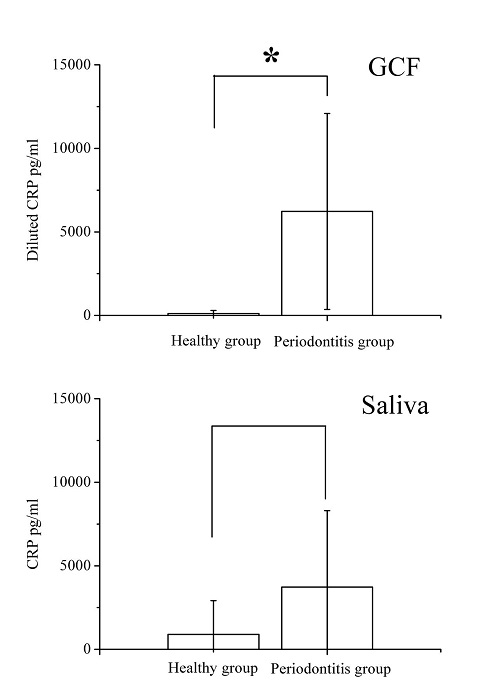
Average C-reactive protein (CRP) concentrations in gingival crevicular fluid (GCF) and saliva: healthy people (SD = 179, n = 14) and periodontitis patients (SD = 5867, n = 11) in GCF, healthy people (SD = 2022, n = 14) and periodontitis patients (SD = 4574, n = 11) in saliva.* shows statistical CRP concentrations difference in GCF between healthy group and patients with periodontitis.
CRP is considered a major marker for systemic acute inflammation in diabetes mellitus and heart disease. (Yuan et al., 2006; Zacho et al., 2008) Elevated levels of CRP are associated with increased risk of diabetes mellitus, ischemic heart disease, and ischemic cerebrovascular disease. A study has linked chronic periodontitis with elevated plasma CRP levels, even after controlling for several potential cofactors.(Gomes et al., 2011) Many dentists and surgeons are concerned about orofacial surgery and oral implant surgery under conditions of acute systemic inflammation because inflammation can decrease the success rate of treatment.
This study involved evaluation of CRP concentrations in saliva and GCF of healthy people and periodontitis patients using an enzyme-linked immunoassay kit. The CRP concentration in periodontitis patients was more sensitively detected in GCF than in saliva. Saliva comes from major and minor salivary glands, which could have been easily stimulated under conditions of saliva collection, diluting the saliva contents.
Recently, some reports have described CRP detection in extra-hepatic regions,(Jabs et al., 2005; Ali et al., 2011) even though CRP is known to be produced in liver cells. But one study involving CRP detection in GCF found that CRP in the GCF appears to be of systemic origin, not of local gingival origin.(Megson et al., 2010) Therefore, CRP in GCF could be considered as an important marker of acute systemic inflammation.
CONCLUSION
In conclusion, the results of this research show detection of CRP in saliva and GCF, and that the CRP concentration in the GCF of periodontitis patients was more sensitively detected than in saliva. In addition, chronic periodontitis patients and healthy controls differed in CRP concentrations in GCF. Therefore, CRP in GCF could be a preferable biomarker for measuring the systemic acute inflammation state in patients with severe periodontitis, which method is considered more comfortable for patients due to their non-puncture procedure.
Acknowledgments
This research was supported by BK21 PLUS Project, College of Dentistry, Yonsei University.
References
-
S Ali, Q Yin-Goen, TV Johnson, W Han, NA Johnson, WB Harris, FF Marshall, AN Young, VA Master, AO Osunkoya, Expression of C-reactive protein and cyclooxygenase enzyme-2 in clear cell renal cell carcinoma: correlation with pathological parameters in 110 patients, Tumour Biol, (2011), 32(2), p375-380.
[https://doi.org/10.1007/s13277-010-0130-9]

-
N Dutzan, R Vernal, M Hernandez, A Dezerega, O Rivera, N Silva, JC Aguillon, J Puente, P Pozo, J Gamonal, Levels of Interferon-Gamma and Transcription Factor T-Bet in Progressive Periodontal Lesions in Patients With Chronic Periodontitis, J Periodontol, (2009), 80(2), p290-296.
[https://doi.org/10.1902/jop.2009.080287]

- IS Gomes, JMF Coelho, SS da Cruz, JS Passos, COT de Freitas, NSA Farias, RA da Silva, MNS Pereira, TL Lima, ML Barreto, Chronic Periodontitis and C-Reactive Protein Levels, J Periodontol, (2011), 82(7), p969-978.
-
WJ Jabs, M Busse, S Kruger, D Jocham, J Steinhoff, C Doehn, Expression of C-reactive protein by renal cell carcinomas and unaffected surrounding renal tissue, Kidney Int, (2005), 68(5), p2103-2110.
[https://doi.org/10.1111/j.1523-1755.2005.00666.x]

-
E Megson, T Fitzsimmons, K Dharmapatni, PM Bartold, C-reactive protein in gingival crevicular fluid may be indicative of systemic inflammation, J Clin Periodontol, (2010), 37(9), p797-804.
[https://doi.org/10.1111/j.1600-051X.2010.01603.x]

-
B Noack, RJ Genco, M Trevisan, S Grossi, JJ Zambon, E De Nardin, Periodontal infections contribute to elevated systemic C-reactive protein level, J Periodontol, (2001), 72(9), p1221-1227.
[https://doi.org/10.1902/jop.2000.72.9.1221]

-
I Ouellet-Morin, A Danese, B Williams, L Arseneault, Validation of a high-sensitivity assay for C-reactive protein in human saliva, Brain Behav Immun, (2011), 25(4), p640-646.
[https://doi.org/10.1016/j.bbi.2010.12.020]

-
S Paraskevas, JD Huizinga, BG Loos, A systematic review and meta-analyses on C-reactive protein in relation to periodontitis, J Clin Periodontol, (2008), 35(4), p277-290.
[https://doi.org/10.1111/j.1600-051X.2007.01173.x]

-
MB Pepys, GM Hirschfield, C-reactive protein: a critical update, J Clin Invest, (2003), 111(12), p1805-1812.
[https://doi.org/10.1172/JCI200318921]

-
LM 1Shaddox, J Wiedey, NL Calderon, I Magnusson, E Bimstein, JA Bidwell, EF Zapert, I Aukhil, SM Wallet, Local Inflammatory Markers and Systemic Endotoxin in Aggressive Periodontitis, J Dent Res, (2011), 90(9), p1140-1144.
[https://doi.org/10.1177/0022034511413928]

-
J Silness, H Löe, Periodontal Disease in Pregnancy II. Correlation Between Oral Hygiene and Periodontal Condition, Acta Odontol Scand, (1964), 22(1), p121-135.
[https://doi.org/10.3109/00016356408993968]

-
E Sjogren, P Leanderson, A Kristenson, J Ernerudh, Interleukin-6 levels in relation to psychosocial factors: Studies on serum, saliva, and in vitro production by blood mononuclear cells, Brain Behav Immun, (2006), 20(3), p270-278.
[https://doi.org/10.1016/j.bbi.2005.08.001]

-
M Tishler, I Yaron, I Shirazi, Y Yossipov, M Yaron, Increased salivary interleukin-6 levels in patients with primary Sjogren's syndrome, Rheumatol Int, (1999), 18(4), p125-127.
[https://doi.org/10.1007/s002960050070]

-
TA Turvey, WP Proffit, C Phillips, Biodegradable fixation for craniomaxillofacial surgery: a 10-year experience involving 761 operations and 745 patients, Int J Oral Maxillofac Surg, (2011), 40(3), p244-249.
[https://doi.org/10.1016/j.ijom.2010.11.024]

-
GY Yuan, LB Zhou, JF Tang, Y Yang, WQ Gu, FY Li, J Hong, YY Gu, XY Li, G Ning, MD Chen, Serum CRP levels are equally elevated in newly diagnosed type 2 diabetes and impaired glucose tolerance and related to adiponectin levels and insulin sensitivity, Diabetes Res Clin Pract, (2006), 72(3), p244-250.
[https://doi.org/10.1016/j.diabres.2005.10.025]

-
J Zacho, A Tybjaerg-Hansen, JS Jensen, P Grande, H Sillesen, BG Nordestgaard, Genetically elevated C-reactive protein and ischemic vascular disease, N Engl J Med, (2008), 359(18), p1897-1908.
[https://doi.org/10.1056/NEJMoa0707402]

