
Cytotoxicity of dental light-cured calcium hydroxide cements on human dental pulp stem cell
This study examined the color changes in a resin composite with different shades after exposure to different drink media. A nanohybrid resin composite (Filtek Z350 XT) was exposed to a pH 6 solution for 2 weeks and then immersed in a staining solution (Coffee or Grape juice) or distilled water (as control) for 3 days. The color coordinates of the specimen before and after immersion in the staining solutions were measured using a spectrophotometer. The color difference (ΔE) and the change in the translucency parameter (ΔTP) were calculated using the CIEL*a*b* system. The data was statistically analyzed using ANOVA and a Tukey’s test. The ΔE of the specimens tested differed significantly according to the staining solutions (p<0.05) and the shades of the composite resins (p<0.05). Coffee and B1 shade showed the greatest color change. The values of TP after immersion in the staining beverages decreased except for distilled water. The absolute ΔTP values of grape juice were higher than the others regardless of the shades. Overall, the color stability of the resin composites differs according to the staining media used and shade of the composite resins.
Keywords:
Color stability, composite resin, staining solution, translucency parameter, resin shadeINTRODUCTION
The main purpose of restorative dentistry is to protect and re-establish pulp function and to restore and maintain tooth health by an adequate restorative treatment (Modena et. al., 2009). Pulp capping is a common technique to preserve pulp vitality of teeth damaged by various causes such as caries and traumatic injury and to protect the dentin-pulp complex. There are two main types of pulp capping methods: indirect and direct pulp capping, in which a specific material is in directly and indirectly contact with the pulp tissue, respectively, when placed between the restorative material and dental tissue. Ideally, pulp capping materials should be biocompatible and have satisfactory physicochemical properties. The proper choice and use of pulp capping materials is essential in the procedures (Cavalcanti et al., 2005).
Calcium hydroxide (CH) has long been used for both indirect and direct pulp capping mainly due to its inherent high pH (Kitasako et al., 2000; Cavalcanti et al., 2005), which can inhibit bacterial growth and recover of acidic tissue pH caused by the action of the bacterial metabolism (Murray et al., 2002). Such CH-based capping agents are available in many forms: CH suspensions, self-cured calcium-salicylate cements, or light-cured CH cements. CH suspension has been commonly utilized as an intracanal dressing rather than a pulp capping material because they do not set and, as a result, have a very low compressive strength (McCabe et al., 2008; Schmalz et al., 2009). Thus, most CH cavity liners in current use are two-component hand-mixed systems. The setting process for the materials is believed to involve a chelating reaction between the zinc oxide (Van Noort, 2013) or CH (Craig et al., 1993) and disalicylate. Thus, self-cured CH cements generally need hand-mixing, and set materials are mechanically weak and soluble over time (Stanley et al., 1985; Cavalcanti et al., 2005).
Light-curable CH cements are more user-friendly because they are one-component systems. They set on command by polymer formation with the use of a dental curing light and provide easier control of the working time (Stanley et al., 1985). A light-cured CH material consists of CH dispersed in diluent monomers such as diurethane dimethacrylate (UDMA) and triethylene glycol dimethacrylate (TEGDMA), together with camphoroquinone (CQ)/amine photoinitiation system (Stanley et al., 1985). UDMA contains two methacrylate double bonds, which allow a high degree of crosslinking and improve physical/mechanical properties (Cao et al., 2011). TEGDMA increases the degree of conversion and crosslinking mainly due to its low molecular weight, lower viscosity and higher reactivity (Sideridou et al., 2002)
Light-cured CH products may maintain all the characteristics of healing and bridge formation equivalent to the original self-cured version (Stanley et al., 1985). However, the most desirable properties of light-cured CH cements containing UDMA and TEGDMA can probably be obtained only when the materials are properly light-cured to optimize the polymerization of the resin matrix. In general, the monomer to polymer conversion of direct resin composites varies between 40% and 75%, indicating an incomplete “intraoral” polymerization (Anusavice et al., 2012). In particular, the adequate light-irradiation of light-cured CH agents is not always possible mainly due to the geometry of the tooth or cavity to be treated, especially in the floor of deep cavities (Albers et al., 2002). In those cases, unreacted UDMA or TEGDMA monomers eluted from the poorly-cured polymer matrix can cause an adverse effect on the pulp tissue. Although previous in vitro studies have demonstrated direct cytotoxic effects of UDMA and TEGDMA monomers to various cell types (Stanislawski et al., 2003; Accorinte et al., 2002; Janke et al., 2003; Schweikl et al., 2007), to our knowledge, only few studies have been carried out to investigate the cytotoxicity of light-cured CH pulp capping materials containing such monomers on pulp tissue.
The dental pulp contains adult stem cells to repair pulp and surrounding dentin. Dental pulp stem cells (DPSCs) are multipotent stem cells, which have the potential to differentiate into a variety of cell types. Recent studies have demonstrated that human DPSCs have the ability to form a dentin/pulp-like complex (Gronthos et al., 2000). Therefore, it is important to evaluate whether CH materials exert some cytotoxicity towards DPSCs. In addition, since direct pulp capping materials are in “direct” contact with pulp tissue for a long time of service, the biocompatibility is of particular importance.
The purpose of this study was to evaluate in vitro cytotoxicity of light-cured CH pulp capping materials, which were light-irradiated with two different light irradiation times, on DPSCs. The degree of conversion (DC) of the materials and the amount of unreacted monomers eluted from the materials were measured using Fourier transform infrared (FTIR) spectroscopy and high performance liquid chromatography (HPLC), respectively. The viability of the DPSCs was investi- gated using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. At each incubation time, in addition, the pH of the cultured medium was measured.
MATERIALS AND METHODS
Specimen preparation
In this study, two commercial light-cured CH cements (Ultra-Blend Plus, U; Calcimol LC, C) containing UDMA and TEGDMA monomers were investigated. A selfcured CH cement (Dycal, D) was also used for comparison. Their detailed information is summarized in Table 1.
Silicone molds for the preparation of specimens with a dimension of 60 mm × 10 mm × 1 mm were placed on a polyester strip over a glass slide (ISO 10993-12, 2002E). The cements were prepared in accordance with the manufacturers’ instructions, and placed in the molds, covered with another polyester strip and glass slide, and gently pressed to expel the excess material. The D cement was then allowed to self-cure. The other two light-cured cements were light irradiated either for 6 s (groups U1 and C1) or 60 s (groups U2 and C2) by placing the tip of the light guide of a dental curing light (Elipar TriLight, 3M EPSP, Seefield, Germany; standard mode) and moving along the entire length of the specimen. The output intensity of 750 mW/cm2 was constantly monitored during the experiment by a built-in radiometer.
Isolation of DPSCs
Non-carious human third molars or premolars were extracted either for surgical or orthodontic reasons with patients’ informed consent under a protocol approved by the Institutional Review Board, Kyungpook National University Hospital. DPSCs were isolated as described previously (Gronthos et al., 2000; Chun et al., 2011). Briefly, teeth were split with a dental engine and the pulp tissues were collected with a periodontal curette, minced into small pieces by a surgical knife, and incubated the shaking incubator with 3 mg/mL of collagenase type I (Sigma Aldrich, St. Louis, MO, USA) and 4 mg/mL of dispase II (Gibco, Scotland, UK) for 1 hr at 37 °C. The tissues were then diluted with α-MEM media and applied to the centrifugation for 10 min. Single-cell suspensions obtained by passing the cells through a 70-μm strainer grown to confluence in α-MEM supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), streptomycin (100 mg/mL). DPSCs were cultured in an incubator at 37°C and a humidified 5% CO2 atmosphere for 10-14 days. Cells within 10th passages were used in all experimental procedures. When the cells reached confluence, a trypsin-EDTA (0.5 g/L trypsin and 0.2 g/L EDTA; Gibco) was used to detach cells from the bottom of the culture dishes and were transferred into a new tissue culture dishes.
MTT assay and pH measurement
Each specimen was immersed in 5 mL of α-MEM supplemented with 10% FBS and antibiotics in conical tube and stored in a incubator at 37°C for 24 h. The medium was filtered with 0.2 μm pore size filter and diluted to ten times using α-MEM medium (Cavalcanti et al., 2005). Cell viability was determined by measuring the viable cells react with MTT. The cells were plated to 48-well culture plates at a density of 1 × 103 cells. After incubation for 24 h, the cells were cultured with α-MEM medium eluted from the specimens prepared as described above and incubated for 6, 24, 96 and 144 h. A filtered MTT solution (5 mg/mL, Sigma Aldrich) was added to each well, and the human DPSCs were incubated at 37°C for 4 h. After removal of the culture medium, dimethylsulfoxide (DMSO) was added to each well to dissolve the cells. Cytotoxicity was assessed by measuring the optical density of the colored product in the solution using a spectrophotometer (Sunrise Absorbance Reader, Tecan Austria GmbH, Salzburg, Austria) at 570 nm. These experiments were run in triplicate or quadruplicate and repeated three separate times to ensure reproducibility (total 10 times). At each incubation time, 300 μL of cultured medium was transferred to e-tube, then the pH was measured using a pre-calibrated digital pH meter (D-51, Horiba Ltd., Kyoto, Japan) with a micro tube glass electrode (9669-10D, Horiba Ltd.). Three readings were taken for each solution, from which the mean pH value was calculated.
DC of the light-cured cements
The DC of the light-cured cements was determined using a FTIR spectroscope (IRPrestige-21, Shimadzu Corp, Kyoto, Japan) with an attenuated total reflectance (ATR) unit (MIRacle, Pike Technologies Inc, Madison, WI, USA). Prior to analysis, an adhesive tape was placed around the ATR diamond surface to act a vertical spacer, ensuring standard specimen thickness (approximately 100 μm). A small amount of each material was pressed against the diamond surface and covered with a polyester strip and glass slide, then light-cured with the same irradiation conditions as for specimen preparation (n = 5). The absorbance spectrum was acquired by scanning the specimens 10 times over a 1815–1380 cm−1 range at a resolution of 4 cm−1. The DC was determined from the aliphatic C=C peak at 1638 cm−1, while the amide II C=C peak at 1537 cm−1 was used as the internal reference (Guerra et al., 1996) The DC was then calculated by comparing the height of the peaks for the methacrylate vinyl group in the cured material against that in the uncured material, using the following formula: DC (%) = 100[1 − (Ha(alipathic C=C)Hb(amide II C=C)/Hb(alipathic C=C) Ha(amide II C=C))], where Ha and Hb are the absorption peak heights after and before light-curing, respectively.
Eluted monomers from the light-cured cements
Each specimen prepared as described above was put into 5 mL HPLC grade water and incubated for 24 h at 37°C. To measure the amount of eluted UDMA and TEGDMA monomers, which are main resin monomers of the two light-cured materials (Table 1), HPLC (LC-20AD, Shimadzu Corp., Kyoto, Japan), which consisted of a reverse-phase column, was used. The mobile phase was a mixture of water/acetonitrile (30: 70). The flow rate was 0.8 mL/min, the injection volume 10 μL, and components were detected at 208 nm [17]. The concentrations of the eluted two monomers were determined using a standard graph established from known concentrations of each corresponding monomer.
Fluorescent microscopic observation
The human DPSCs were cultured at density of 5 × 104 per 12-well in chamber slide. After incubation for 24 h, the medium were changed with medium eluted from the specimens prepared as described above and the cells were cultured for 6 days at 37 °C in 5% CO2 incubator. After 144 h in culture, adherent cells were fixed and stained with 5 μg/mL of DAPI (4',6-diamidino-2-phenylindole, Vector Laboratories Inc., Burlingame, CA, USA) and observed using fluorescence microscope (Leica DM IL LED, Germany), and DAPI-positive cells were counted under fluorescent microscope at 100× magnification. The cells were counted in different 3 areas in well plate.
Statistical analysis
For MTT, FTIR, and HPLC data were statistically analyzed by 1-way ANOVA with Tukey’s post hoc test at a significance level of 0.05, using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
DPSCs reside within the perivascular niche of dental pulp and have the ability to form a dentin/pulp-like complex (Arthur et al., 2008). In the current study, therefore, DPSCs derived from human tooth were used to investigate the cytotoxicity of light-cured CH cements on pulp tissue to elucidate the effect of CH cement on dental tissue. The two light-irradiation time conditions employed in this study were to simulate an insufficient light-curing condition and to prepare specimens following the ISO 10993-12 (6 s) or to ensure the maximum polymerization of the materials (60 s). In a pilot test, all media eluted from the CH cements proved to be highly cytotoxic to the DPSCs. Thus, it was decided to work with this medium diluted to 10%, according to Cavalcanti et al. (2005) in order to make this comparative study possible. This dilution seems appropriate since the cell numbers are higher than the number of cells in a culture dish in pulp tissue. At the same time, blood and lymphatic vessels are present in living tissue, which dilute the substances (Cavalcanti et al., 2005)
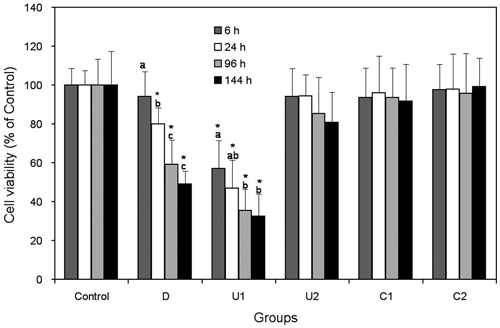
Cell viability of human DPSCs for each group (against control) as a function of incubation time. Each bar represents a mean ± SD. * denotes significant differences from Control within the same incubation time (p < 0.05, 1-way ANOVA and Tukey’s test). The same letters above bars indicate that statistically similar values within the same experimental group (p > 0.05).
Figure 1 represents the cell viability of human DPSCs for each group against the control, and the results show that the light-irradiation time of light-cured CH cements affect the cytotoxicity to DPSCs, but with material-specific. Group U1 showed significantly lower cell viabilities as compared to the control at all incubation time conditions (p < 0.05). The other three light-cured groups (U2, C1, and C2) did not show any significant differences in cell viability regardless of the incubation time (p > 0.05). The self-cured material, D, also showed a significant toxicity to DPSCs at 24, 96, and 144 h.
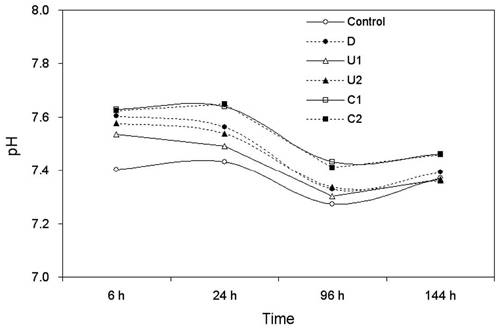
Changes in pH values for each group when incubated with medium containing eluted monomers for a definite time.
Figure 2 shows the pH changes for the control and five experimental groups during the experimental period investigated to determine whether the pH affected the cytotoxicity results shown in Fig. 1. In general, lightcured CH materials show a pH of 11–12 before polymerization (Schmalz et al., 2009). In this study, the values were slightly alkaline but remained within a narrow range of 7.3–7.6 during the experimental period, probably by the reaction that calcium hydroxide reacts with sodium bicarbonate producing sodium hydroxide and calcium carbonate (Andreola et al., 2007): Ca(OH)2 + Na2CO3 → 2NaOH + CaCO3. This finding experimentally verifies the buffer action of the sodium bicarbonate of the medium, which were only assumed in previous cytotoxicity studies (Cavalcanti et al., 2005; Medina et al., 2002). Thus, the influence of pH on the cytotoxicity appears minimal within the present experimental set-up, and can be assumed that some cytotoxicity was caused probably by eluted resin monomers (light-cured material) or other toxic substances (self-cured material) (Table 1), rather high alkalinity caused by CH.
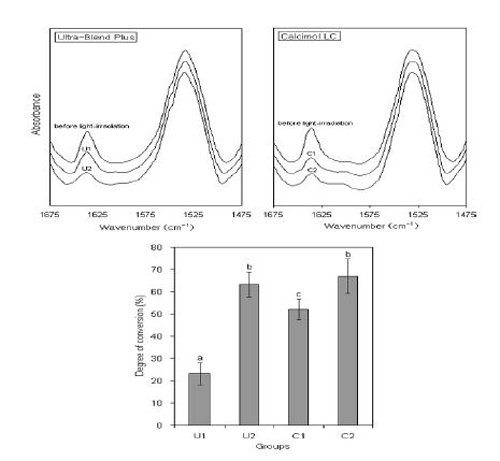
FTIR spectra normalized against the amide II C=C peak at 1537 cm−1 and degree of conversion for the four experimental groups. Each bar represents a mean ± SD. The same letters above bars indicate that statistically similar values. (p > 0.05, 1-way ANOVA and Tukey’s test).
Figure 3 clearly shows that the two light-cured materials have different light-curability. DC of a lightcured material can be determined by its composition as well as the clinical usage (Kwon et al., 2012). When the materials were light-irradiated for a short time, C1 exhibited a significantly higher DC (52.1%) than U1 that showed a very low value of 23.2% (p < 0.001). When light-irradiation was performed for 60 s, on the other hand, no significant difference in the value was found between the two materials (p = 0.741). The DC values of light-cured CH cements light-irradiated for 60 s appear similar to those of light-cured dental composites (Anusavice et al., 2012). These findings also imply that unreacted monomers elute from lightcured CH materials even when the materials are properly light-irradiated.
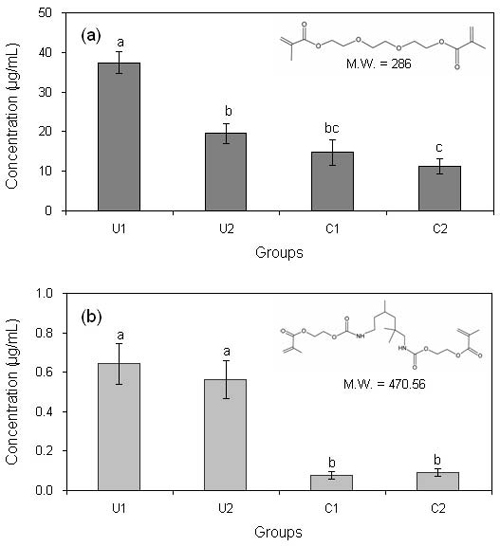
Concentrations of TEGDMA (a) and UDMA (b) resin monomers released from the light-cured materials. The same letters above bars indicate that statistically similar values (p > 0.05, 1-way ANOVA and Tukey’s test).
The concentrations of TEGDMA and UDMA resin monomers released from the four light-cured materials are depicted in Figure 4. The two monomers are common matrix monomers of dental composites or dentin adhesives (Ruyter et al., 1981). It has been shown that unreacted TEGDMA or UDMA elute from the cured dental resin-based materials into the adjacent aqueous phase and diffuse through dentin to the pulp space (Gierzina et al., 1996). Recent studies show that these residual unpolymerized monomers released, in particular from dentin adhesives that are placed directly on dentin or pulp, are responsible for pulpal diseases such as pulp inflammation (Costa et al., 2000; Pereira et al., 2000). The HPLC results of this study were consistent with those of FTIR spectroscopy (Figure 3). The highest concentration of TEGDMA released was found in group U1, which showed the lowest DC. Fig. 4 also implies that the cytotoxicity shown in group U1 (Figure 1) was primarily caused by a high concentration of TEGDMA released from the poorly-cured material rather than UDMA when the material was not adequately light-cured. The higher molecular weight and more complex molecular structure of UDMA may have made the release from the cross-linked resin matrix formed by photopolymerization difficult when compared to TEGDMA (Figure 4) (Craig et al., 1993). It has been reported that TEGDMA inhibited the gingival cell proliferation and cause the apoptosis (Janke et al., 2003). The TC50 values of UDMA and TEGDMA range from 0.06 to 0.47 mM and from 1.2 to 2.6 mM, respectively, depending on cell types and assays (Chang et al., 2010; Stanislawski et al., 2003). Moreover, these monomers have been reported to affect differentiation in various kinds of cells, especially, human pulp cells (About et al., 2002). Thus, in present study demonstrated that toxicity of CH cements as pulp capping materials is depended on eluted monomer concentration and these monomers may inhibit the proliferation of DPSCs.
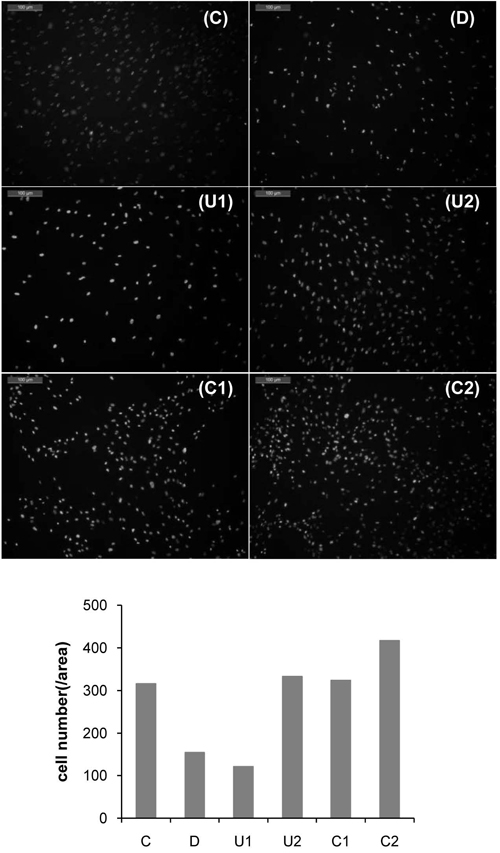
Fluorescent microscopic images of DAPI-stained DPSCs cultured with medium eluted from the CH specimens for 144 h (magnification: 100×) (upper six images). The number of DAPI-positive cells was also counted from the fluorescent image (lower).
Figure 5 shows the cells stained with fluorescence dye, DAPI. In accordance with cell viability result (Figure 1), groups D and U1 represented lower cell counts than the other groups. On the contrary, there was no significant difference in cell count between groups C1 and C2. Janke et al. showed the apoptotic cell death over at least 2.5 mM TEGDMA. The concentration of TEGDMA would be a low concentration to induce apoptotic cell death. In the MTT assay, cells grown in media with C1 did not show cytotoxicity. In the present study, our results indicated that toxicity of DPSCs can be caused by low concentration of eluted monomer (UDMA and TEGDMA) from the light-cured CH cements and such a low concentration of them. But the concentration of TEGDMA was very low.
There have been frequent attempts in the past to replace CH with other substances. Among them, the use of adhesive systems has been suggested as a pulp capping procedure, but the clinical results are still controversial (Cavalcanti et al., 2005; Accorinte et al., 2005). Most recently, mineral trioxide aggregate (MTA), have been highlighted and extensively researched (Schmalz et al., 2009). However, light-cured CH pulp capping agents are still regarded to be the first choice for treating pulp exposure (Cavalcanti et al., 2005; Accorinte et al., 2005). The results of the present in vitro study suggest that, however, light-cured CH cements should be adequately polymerized using sufficient light irradiation times in order to minimize possible adverse cytotoxic effect of the residual resin monomers on pulp cells. This finding should also be verified using in long-term clinical observations as well as in vivo tests.
CONCLUSIONS
In this experimental set-up, the influence of pH of CH-containing resin-based cements seemed negligible probably due to the buffer action of medium containing sodium bicarbonate. The present in vitro study suggests that unreacted monomers such as TEGDMA and UDMA eluted from poorly light-cured resin-based CH cements may cause the pulp cell death, although the light-curability of the materials may differ depending on the brands. In conclusion, light-cured CH pulp capping agents should be adequately light irradiated to avoid the adverse effect of the residual monomers to pulp cells. Such proper light-curing may be effectively obtained by using a dental light curing unit with a high light intensity and longer light irradiation time.
References
- I Accorinte, J Camps, TA Mitsiadis, MJ Bottero, W Butler, JC Franquin, Influence of resinous monomers on the differentiation in vitro of human pulp cells into odontoblasts, J Biomed Mater Res, (2002), 63, p418-423.
-
L Accorinte Mde, AD Loguercio, A Reis, A Muench, VC de Araújo, Adverse effects of human pulps after direct pulp capping with the different components from a total-etch, three-step adhesive system, Dent Mater, (2005), p599-607.
[https://doi.org/10.1016/j.dental.2004.08.008]

- HF Albers, Tooth-Colored Restoratives: Principles and Techniques, ed 9, Hamilton: BC Decker Inc, (2002).
- KJ Anusavice, RW Phillips, C Shen, HR Rawls ed 11, Phillips' science of dental materials, Elsevier Health Sciences, (2012).
-
R Andreola, O Vieira, OAA dos Santos, LMM Jorge, Effect of water losses by evaporation and chemical reaction in an industrial slaker reactor, Braz Arch Biol Technol, (2007), 50, p339-347.
[https://doi.org/10.1590/S1516-89132007000200019]

-
A Arthur, G Rychkov, S Shi, SA Koblar, S Gronthos, Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues, Stem Cells, (2008), 26, p1787-1795.
[https://doi.org/10.1634/stemcells.2007-0979]

-
Z Cao, X Sun, CK Yeh, Y Sun, Effects of methacrylic acid on physical/mechanical properties and biocompatibility of urethane-based denture biomaterials, Materials Sciences and Application, (2011), 2, p1070-1075.
[https://doi.org/10.4236/msa.2011.28144]

- BN Cavalcanti, SM Rode, MM Marques, Cytotoxicity of substances leached or dissolved from pulp capping materials, Int Endod J, (2005), 38, p505-509.
-
HH Chang, MC Chang, LD Lin, JJ Lee, TM Wang, CH Huang, TT Yang, HJ Lin, JH Jeng, The mechanisms of cytotoxicity of urethane dimethacrylate to Chinese hamster ovary cells, Biomaterials, (2010), p6917-6925.
[https://doi.org/10.1016/j.biomaterials.2010.05.059]

-
SY Chun, HJ Lee, YA Choi, KM Kim, SH Baek, HS Park, JY Kim, JM Ahn, JY Cho, DW Cho, HI Shin, EK Park, Analysis of the soluble human tooth proteome and its ability to induce dentin/tooth regeneration, Tissue Eng Part A, (2011), 17, p181-91.
[https://doi.org/10.1089/ten.tea.2010.0121]

- CA Costa, J Hebling, CT Hanks, Current status of pulp capping with dentin adhesive systems: a review, Dent Mater, (2000), 16, p188-197.
- RG Craig, JM Powers 12th Eds, Restorative dental materials (Vol. 184), St. Louis: Mosby, (1993).
- TM Gierzina, WR Hume, Diffusion of monomers from bonding resin–resin composite combinations through dentine in vitro, J Dent, (1996), 24, p125-128.
- S Gronthos, M Mankani, J Brahim, PG Robey, S Shi, Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo, PNAS, (2000), 97, p13625-13630.
-
RM Guerra, I Durán, P Ortiz, FTIR monomer conversion analysis of UDMA-based dental resins, J Oral Rehabil, (1996), 23, p632-637.
[https://doi.org/10.1111/j.1365-2842.1996.tb00903.x]

- ISO 10993-12, Biological evaluation of medical devices ― Part 12: Sample preparation and reference materials, (2002E).
-
V Janke, N von Neuhoff, B Schlegelberger, G Leyhausen, W Geurtsen, TEGDMA causes apoptosis in primary human gingival fibroblasts, J Dent Res, (2003), 82, p814-818.
[https://doi.org/10.1177/154405910308201010]

- Y Kitasako, S Shibata, PN Pereira, J Tagami, Short-term dentin bridging of mechanically-exposed pulps capped with adhesive resin systems, Oper Dent, (2000), 25, p155-162.
-
TY Kwon, R Bagheri, YK Kim, KH Kim, MF Burrow, Cure mechanisms in materials for use in esthetic dentistry, J Investig Clin Den, (2012), 3, p3-16.
[https://doi.org/10.1111/j.2041-1626.2012.00114.x]

- JF McCabe, A Walls 9th Eds, Applied dental materials, John Wiley & Sons, (2008).
- VO Medina 3rd, K Shinkai, M Shirono, N Tanaka, Y Katoh, Histopathologic study on pulp response to single-bottle and self-etching adhesive systems, Oper Dent, (2002), 27, p330-342.
-
KC Modena, LC Casas-Apayco, MT Atta, CA Costa, J Hebling, CR Sipert, MF Navarro, CF Santos, Cytotoxicity and biocompatibility of direct and indirect pulp capping materials, J Appl Oral Sci, (2009), 17, p544-554.
[https://doi.org/10.1590/S1678-77572009000600002]

-
PE Murray, Y Kitasako, J Tagami, LJ Windsor, AJ Smith, Hierarchy of variables correlated to odontoblast-like cell numbers following pulp capping, J Dent, (2002), 30, p297-304.
[https://doi.org/10.1016/S0300-5712(02)00024-6]

- JC Pereira, AD Segela, CA Costa, Human pulpal response to direct pulp capping with an adhesive system, Am J Dent, (2000), 13, p139-147.
-
JE Ruyter, JJ Sjovik, Composition of dental resin and composite materials, Acta Odontol Scand, (1981), 39, p133-146.
[https://doi.org/10.3109/00016358109162272]

- G Schmalz, D Arenholt-Bindslev, Biocompatibility of dental materials Springer, Berlin, Germany, (2009).
-
H Schweikl, A Hartmann, KA Hiller, G Spagnuolo, C Bolay, G Brockhoff, G Schmalz, Inhibition of TEGDMA and HEMA-induced genotoxicity and cell cycle arrest by N-acetylcysteine, Dent Mater, (2007), 23, p688-695.
[https://doi.org/10.1016/j.dental.2006.06.021]

-
I Sideridou, V Tserki, G Papanastasiou, Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins, Biomaterials, (2002), 23, p1819-1829.
[https://doi.org/10.1016/S0142-9612(01)00308-8]

-
L Stanislawski, M Lefeuvre, K Bourd, E Soheili-Majd, M Goldberg, A Périanin, TEGDMA-induced toxicity in human fibroblasts is associated with early and drastic glutathione depletion with subsequent production of oxygen reactive species, J Biomed Mater Res A, (2003), 66, p476-482.
[https://doi.org/10.1002/jbm.a.10600]

- HR Stanley Pameijer, CH Pulp capping with a new visible-light-curing calcium hydroxide composition (Prisma VLC Dycal), Oper Dent, (1985), 10, p156-163.
- R Van Noort 3th Eds, Introduction to Dental Materials4: Introduction to Dental Materials, Mosby Elsevier, (2013).
