Effects of different remineralizing agents on the shear bond strength of universal adhesive on enamel
Abstract
This study aimed to evaluate the effect of different remineralization agents on the shear bond strength (SBS) on enamel and to confirm remineralization capacity through quantitative light-induced fluorescence (QLF). Sixty non-carious human third molars were divided into eight groups based on remineralization agents agents (control, Tooth Mousse plusTM, Apapro, BGS-7 bioglass) and application time (24 h, 2 weeks). Enamel surfaces were prepared and treated with agents, followed by demineralization and remineralization. Quantitative Light-induced Fluorescence (QLF) assessed fluorescence loss and recovery. After adhesive application and composite restoration, shear bond strength (SBS) was measured. Statistical analysis included Shapiro-Wilk, ANOVA, Kruskal-Wallis, paired t-tests, Wilcoxon rank tests, and multiple comparison tests. SBS values did not show a significant difference between the groups according to the type and application time of the remineralization agents. QLF measurement, in the 24 h groups, showed no significant difference in the recovery amount between the groups. In the 2 week groups, a statistically significant difference was observed and the value was significantly higher in the BG group than that in the control group. There were no significant differences in the values based on the application time. Within the limitations of this study, bioactive glass showed higher remineralization ability than all the other experimental agents. The SBS was not affected by the remineralizing agent.
초록
본 연구의 목적은 다양한 재광화제가 법랑질의 전단결합강도(SBS)에 미치는 영향을 평가하고 정량 광유도 형광법(QLF)을 통하여 재광화 능력을 확인하는 것이다. 총 60개의 우식이 없는 인간 제3대구치의 치근을 제거한 후 근원심 방향으로 이등분하고 법랑질 표면이 노출되게 포매한 뒤 법랑질 표면을 사포로 연마하였다. 총 120개의 시편들을 재광화제 종류 [대조군. Tooth Mousse plusTM (TM), Apapro (AP), BGS-7 bioglass (BG)]와 도포 시간(24시간, 2주)에 따라 임의로 8개의 군으로 나누었다. 모든 시편들은 탈회 용액에 37 ℃에서 48시간 동안 담가 탈회시켰고, 그 후 재광화제를 적용하여 simulated body fluid (SBF)에 37 ℃에서 보관하였다. QLF 시험으로 탈회 및 재광화 후의 형광 소실도를 정량적으로 측정하였으며, 측정된 값으로부터 회복량을 계산하였다. 이후 레진 수복하여 법랑질의 전단결합강도(SBS)를 측정하였다. 자료는 Shapiro-Wilk test, 일원 변량 분석, Kruskal-Wallis test, 대응표본 t 검정, Wilcoxon rank test, Scheffe test, Dunn test, Bonferroni 검정으로 분석되었다. 전단결합강도는 적용된 재광화제 종류와 시간에 따라 군 간에 유의한 차이를 보이지 않았다. QLF 측정 결과, 24시간 적용한 경우, 회복량은 실험군 간에 유의한 차이가 없었으나, 2주 적용시에는 통계적으로 유의한 차이가 있었으며, 대조군보다 BG 군에서 더 높은 값을 보였다 (p<0.05). 적용 시간에 따른 값의 유의한 차이는 보이지 않았다. 본 연구의 한계 내에서, bioactive glass는 실험된 다른 재광화제에 비해 높은 재광화능을 보였으며, 전단결합강도는 도포된 재광화제 종류에 영향을 받지 않았다.
Keywords:
Remineralization, Quantitative light-induced fluorescence, Shear bond strength키워드:
재광화제, 정량 광유도 형광법, 전단결합강도Introduction
Demineralization of enamel before the formation of carious cavities that can later develop into advanced carious lesions are early carious lesions. These early carious lesions can be remineralized in several ways. These treatments include active oral health education, dietary control, and application of topical agents (1) containing fluoride, casein phosphopeptide-amorphous calcium phosphate (CPP-ACP), nano-hydroxyapatite (nHA), or bioactive glass (BAG).
CPP-ACP, sourced from casein, a milk protein, has the ability to stabilize calcium phosphate and sustain a state of supersaturation of these ions in the mouth. This leads to increased levels of calcium phosphate in the biofilm, thereby promoting tooth structure improvement and facilitating remineralization (2). Hydroxyapatite (HA), the primary mineral in human bones and teeth, is the most stable calcium-phosphate compound in normal bodily conditions. Nano-hydroxyapatite (nHA) has the capacity to improve remineralization by facilitating the spread of mineral ions deeper into the lesion (3). BAG, an inorganic amorphous, calcium, sodium phospho-silicate material. BAG induces apatite formation on the external enamel surface, thereby leading to enamel remineralization (4).
Early caries with a possibility of remineralization can be treated at the time of maintenance or recall visits using various remineralization agents, and the loss of tooth structure can be minimized. When a cavitation lesion progresses, restoration with a resin composite may be necessary, potentially requiring bonding to the remineralized enamel. Regarding the bond strength after application of remineralization agent, some studies have reported that remineralization agent increases the resistance of enamel to acid and decreases the bond strength (5, 6). However, some studies have demonstrated that remineralization agents do not affect the bond strength to enamel (7-9), while others reported that they increase the bond strength (10-12). Those results might be varied depending on the kind of remineralizing agents and the application time or methods. In this study, we investigated the several remineralizing agents that can be supplier of Ca and P on demineralized enamel. Additionally, this is the first try to apply BGS-7 (CGBIO, Seoul, South Korea), a new bioactive glass, as reminerlizing agent on the tooth.
The purpose of this study was to evaluate the effect of different remineralization agents on the shear bond strength (SBS) of resin composite (Filtek Z350 XT Universal, 3M ESPE, St. Paul, MN, USA) to enamel and to confirm the remineralization capacity through quantitative light-induced fluorescence (QLF).
Materials and Methods
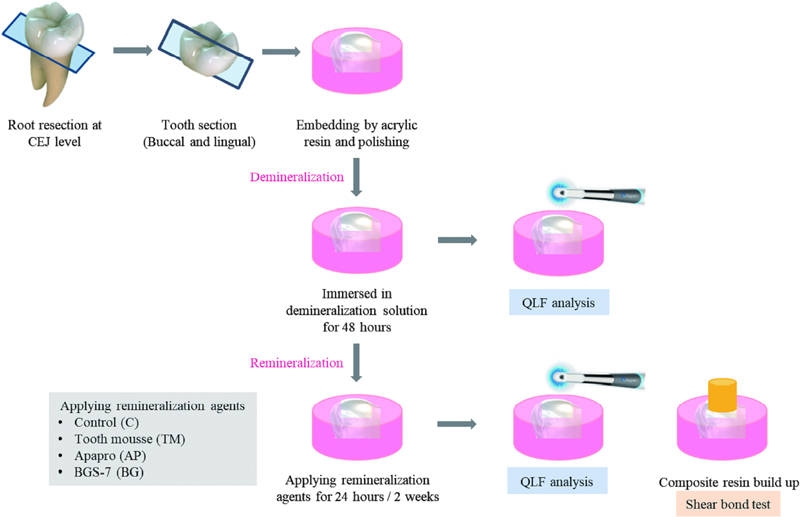
1. Preparation of specimens
This study was approved by the Institutional Review Board of Wonkwang Dental Hospital, Wonkwang University, Iksan, Korea (WKDIRB202403-01). Sixty extracted non-carious human third molars were selected for this study. The extracted teeth were washed to remove debris and immersed in distilled water until further use. The roots of the teeth were removed at the cementoenamel junction and the crown was sectioned mesiodistally into two parts. By cutting the crown mesiodistally, it was possible to obtain two specimens from one tooth. A total of 120 specimens were fabricated using 60 teeth. The sections were placed in cylindrical molds and embedded in acrylic resin (Ortho-Jet, Lang Dental Manufacturing Co., Wheeling, IL, USA) showing the exposed enamel surface. The enamel surface was ground flat and polished with water-cooled 200-, 400-, 600-, 800-, and 1000-grit silicon carbide papers (DEERFOS Co., Seoul, Korea).
2. Demineralization and remineralization protocol
Demineralization was performed by immersing teeth in demineralization solution composed of 2.2 mM calcium chloride (CaCl2·2H2O), 2.2 mM potassium phosphate monobasic (KH2PO4), and 50 mM acetic acid (pH=4.4) (Biosesang, Seongnam, Korea) at 37 ℃ for 48 h (13).
For the remineralization protocol, sufficient amount of each remineralizing agent were spread onto the enamel and applied for 5 minutes by a rubbing motion with a micro brush. For the TM and AP groups, the paste were used, and for the BG group, BG was applied as 10 wt% suspension (One-tenth of a gram of BG powder with 0.9 g distilled deionized water). At the end of the application, the agents were removed by brushing with a toothbrush for 15 s. The remineralization procedure was conducted once a day for 1 time to 14 times along the groups.
All specimens were stored in simulated body fluid (SBF) (pH 7.4, composed of BACl, K2HPO4·3H2O, MgCl2·6H2O, CaCl2, Na2SO4, and NH2C(CH2OH)3) at 37 ℃. The SBF was renewed daily until adhesion was achieved.
3. Experimental groups
The prepared one hundred and twenty specimens were randomly divided into eight groups based on the remineralizing agent and application time (n=15).
(1) Control (C). no treatment for 24 hours; (2) C for 2 weeks; (3) Tooth Mousse plusTM (TM; Recaldent, GC Corp., Tokyo, Japan) for 24 hours; (4) TM for 2 weeks; (5) Apapro (AP; SANGI Co., Tokyo, Japan) for 24 hours; (6) AP for 2 weeks; (7) BGS-7 bioglass (BG; CGBIO, Seoul, South Korea) for 24 hours; (8) BG for 2 weeks.
4. QLF measurement
Five specimens from each group were used for QLF to measure the demineralized areas of the specimens after demineralization and remineralization. All specimens were assessed using a Qraypen C device (AIOBIO, Seoul, Korea) based on QLF technology. Before imaging, the specimens were washed with distilled water and sufficiently dried using compressed air for 5 s, after which imaging was performed. The Qraypen C device was applied vertically to the surface of specimens and the images were taken at the focusing area within the range of 5 mm to 45 mm.
The amount of fluorescence loss of the specimen was measured using Qray software (AIOBIO) by measuring ΔF (%), which indicates the amount of fluorescence loss compared to the demineralized surface (no treatment) in the fluorescence image taken. Recovery amount was calculated as follows:
The recovery amount(Δ(ΔF)) = ΔFremin -ΔFdemin
5. Bonding procedure
The surfaces of all the teeth were dried. Etching was performed using 37% phosphoric acid (DenFil Etchant, 37%; Vericom, Anyang, Korea). The etchant was washed with an air-water spray for 10 s and dried until the surface was white and chalky. All Bond Universal (BISCO, Schaumburg, IL, USA) was used with a microbrush, and the surface was agitated for 20 s. The solvent was air-dried for 10 s and polymerized using a light emitting diode (LED) curing unit (Elipar s10, 3M ESPE, St. Paul, MN, USA) at an output intensity of 1,100 mW/cm2 for 10 s. The composite resin (Filtek Z350 XT Universal, 3M ESPE) was placed in two 2 mm-thick layers using a cylindrical plastic tube of 3.2 mm inner diameter and height of 3 mm. The composite resin layer was polymerized for 20 s. And then the cylindrical plastic tube was removed carefully. Bonding procedure for shear bond strength test was performed according to ISO 29022:2013. The specimens were then incubated at 37 ℃ in distilled water for 24 h.
6. Shear bond strength test
The micro-SBS test was performed using a shear bond tester (T-63010K , BISCO) at a cross head speed of 0.5 mm/min. The maximum force for debonding was recorded in Newtons (N) and calculated to MPa by dividing it by the bonding area (N/mm2).
7. Statistical analysis
The Shapiro-Wilk test was performed for normality. One-way analysis of variance (ANOVA) and Kruskal-Wallis test were used to compare the recovery amount and SBS of the specimens between the experimental groups. Paired t- and Wilcoxon rank tests were used to compare the values at 24 h and 2 weeks within the group. Scheffe and Dunn tests were used as post-hoc tests. Bonferroni correction was used to adjust the p-value. All statistical analyses were performed using SPSS v28.0 (IBM Corp.; Armonk, NY, USA).
The experimental procedures and materials tested are summarized in Figure 1 and Table 1.
Results
1. QLF measurement
The results of the QLF measurements from all the experimental groups are shown in Table 2. In the results of groups of 24 h, the recovery amount were significantly different among the four groups (p = 0.036). However, when a post-hoc test was performed using the Dunn test, and the p-value was corrected using the Bonferroni method, no significant difference was found. In the 2 weeks groups, a statistically significant difference was observed in the recovery amount. This value was significantly higher in the BG group than that in the control group (p<0.05).

Mean amount of fluorescence loss (ΔF) and recovery amount (Δ (ΔF)) of the experimental groups (means±S.D)
Comparison over the time of remineralizing agents application within each group, yielded no significant difference in the recovery amount between the 24 h and 2 weeks groups.
The representative images of the experimental groups are shown in Figures 2 and 3. In Figure 2., all specimens showed a light red fluorescence. Any differences were not observed between the demineralized surfaces (A, B, C and D) and 24 h groups (a, b, c and d). In the image of BG 2 weeks group (Figure 3d), it seemed that the red fluorescent area were slightly decreased compared to the image of Figure 3D.
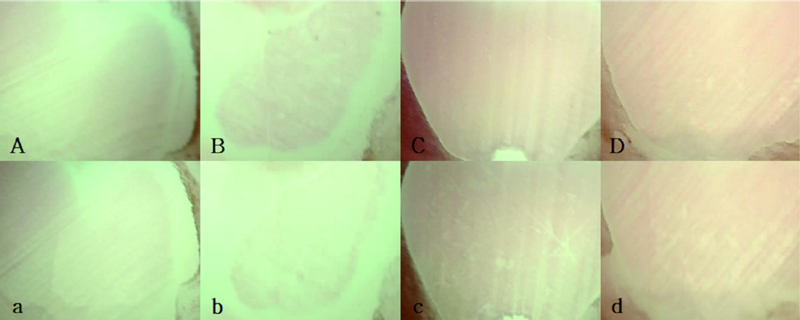
Representative QLF images of 24 h groups.A,a : Control ; B,b : Tooth Mousse plusTM (TM) ; C,c : Apapro (AP) ; D,d : BGS-7 bioglass (BG). QLF Images after exposure in a demineralization solution (A, B, C, D) and after application of remineralizing agents (a, b, c, d) All samples exhibited a faint red fluorescence. No distinctions were noted between the demineralized surfaces (A, B, C, and D) and the 24-hour groups (a, b, c, and d).
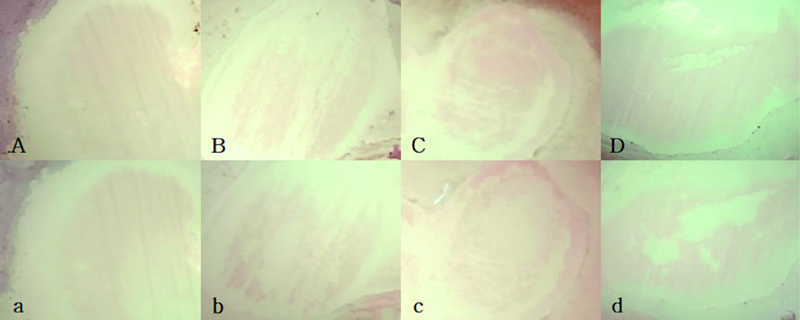
Representative QLF images of 2 weeks groups.A,a : Control ; B,b : Tooth Mousse plusTM (TM) ; C,c : Apapro (AP) ; D,d : BGS-7 bioglass (BG). QLF Images after exposure in a demineralization solution (A, B, C, D) and after application of remineralizing agents (a, b, c, d). In the BG 2 weeks group image (Figure 3. d), it appeared that the area of red fluorescence had slightly diminished compared to the image in Figure 3.D.
2. Shear bond strength test
The results of the SBS tests for all the experimental groups are listed in Table 3. There was no statistically significant difference in the SBS among the groups pretreated with TM, AP, and BG at both 24 h and 2 weeks. Regarding application time, none of the experimental groups showed significant differences between 24 h and 2 weeks.
Discussion
Hypomineralized enamel is more porous than sound enamel because of its lower mineral content. Thus, bonding to this type of enamel may be weak and accompanied by extensive microleakage (14). However, in early enamel caries, lost minerals can be re-supplied through the remineralization process. As calcium and phosphate ions play essential roles in remineralization, novel materials containing these ions have been introduced to the market to prevent and treat enamel hypomineralization. The most popular remineralizing products are CPP–ACP and HA (15). Furthermore, a powerful remineralizing agent primarily consisting of BAG was introduced, demonstrating the ability to enhance the mechanical properties of demineralized enamel in a relatively brief period (16). Traditionally, fluoride products have been widely used for the remineralization of early enamel lesions. However, due to the controversy surrounding the potential harm of fluoride to the human body, there is a tendency for patients to be wary of fluoride products. Both Tooth Mousse Plus, containing CPP-ACPF, and Apapro, containing nHA, serve as remineralizing agents that can be used for the prevention of early dental caries lesions. They both aid in the remineralization of teeth by forming or supplying mineral structures consisting of calcium and phosphate. There is limited research on BGS-7 as a restorative material compared to other BAGs, so we conducted our study using BGS-7. Therefore, the objective of this study was to assess the remineralization effectiveness of these agents and their impact on bonding to treated enamel lesions. To achieve this goal, early enamel carious lesions were artificially induced on the enamel surfaces of the specimens, followed by treatment with chosen remineralizing agents.
Nanohydroxyapatite is gaining attention for preventing cavities by restoring minerals to damaged enamel and dentin after decay. In early decay, acidic bacteria cause mineral loss while leaving collagen intact. Nanohydroxyapatite replenishes minerals directly or as a carrier, aiding remineralization. It's often in toothpaste to promote this. Applied to teeth, synthetic nanohydroxyapatite nanoparticles form a protective layer by penetrating the surface pores (17).
CPP-ACPF delivers not only calcium and phosphate ions but also supplementary fluoride for remineralization. The combination of CPP-ACP with fluoride is likely to bring about the co-localization of calcium, phosphate, and fluoride ions on the enamel surface. Consequently, it provides all the necessary ions for the formation of fluoroapatite crystals, which offer greater resistance to cavities (18).
BGS-7, categorized as bioactive glass, is composed of amorphous sodium-calcium-phosphosilicate. This ceramic material exhibits high reactivity in water and, when finely powdered, can physically block dentinal tubules (19).
The QLF was used to assess the enamel remineralization capacity of the agents tested in this experiment. Surface microhardness tests and observations using polarizing microscope or electron microscopes have been proposed to measure the enamel remineralization effect. These traditional methods are invasive, and can be used in experiments. However, QLF is non-invasive method and can be a useful tool for measuring the degree of remineralization in the clinic.
QLF is a technology that can non-invasively detect and quantitatively analyze early carious lesions through differences in the autofluorescence response of teeth using light in the blue visible light region (20). The equipment is widely used not only in laboratories but also in clinical settings. QLF is useful for clinicians to detect the presence of and changes in early carious lesions because it is possible to detect and monitor micro changes in minerals without tooth destruction (21).
In this study, a recovery tendency was observed in the control and experimental groups that used remineralization agents. Comparing the results according to the type of remineralization agents, the amount of recovery was not significantly different between the 24 h groups. However, the 2 weeks groups showed a statistically significant difference in remineralizing ability, and the value was higher in the BG group than that in the control group.
Bioactive glass (BAG) serves as a biomimetic mineralizer, mirroring the body's natural mineralization processes and influencing cell signaling, ultimately fostering the repair of tissue structure and function. In the aqueous surroundings near the tooth, such as saliva in the mouth, sodium ions from the BAG particles swiftly replace hydrogen ions (H3O+), leading to the liberation of calcium and phosphate ions (PO4-) from the glass material (22). Upon the material's first contact with water, there is a brief, localized rise in pH resulting from sodium release. This pH elevation facilitates the precipitation of excess calcium and phosphate ions from the BAG, forming a calcium phosphate layer. Over time, this layer undergoes crystallization, ultimately transforming into hydroxycarbonate apatite (HCA).
These particles adhere to the tooth surface, persistently emitting ions, and subsequently aid in the remineralization of the tooth surface following the initial application. The deposits are firmly attached and could not be removed by thorough washing and brushing. In in vitro studies, these particles were shown, to release ions and transform into HCA for up to 2 weeks (23).
Another reason why BG showed significant remineralization ability than the control group compared with other remineralizing agents could be that it has different properties from the other materials. Zhou et al. (24) examined the ability of BAG and CPP-ACP to promote remineralization through scanning electron microscopy. They observed that BAG effectively seals the fissures created after demineralization with a closer fit, resulting in more angular deposits, whereas CPP-ACP forms smaller and less defined deposits with an amorphous structure. Unlike BAG, which attaches to the tooth surface and promotes prolonged remineralization, CPP-ACP does not adhere to enamel and therefore does not support long-term tooth surface remineralization (25). The mineral penetration capacity of HA varies according to pH levels; a decrease in pH from 7.0 to 4.0 results in greater mineral deposition within the inner portion of the lesion (26). This may also be directly related to the solubility of nano HA increased in the acidic condition (27). Multiple studies have shown that acidic solutions have a higher ability to facilitate the penetration of mineral ions during remineralization compared to neutral solutions. In the case of BAG, more minerals were deposited under neutral conditions than under acidic conditions (26). Elevated alkalinity influences the dissolution and remineralization rates, potentially attributed to the elevated pH enabling a greater silica concentration in the solution. It is suggested that BGS-7, possessing elevated levels of calcium and phosphorus along with finely sized particles, may produce outstanding outcomes when used as a remineralizing agent.
Clinically, the bonding procedure can often be applied to demineralized or remineralized enamel. Early carious enamel lesions, especially white spot lesions, are common unintended side effects of orthodontic treatment using fixed equipment. For the management of whit-spot lesions, remineralization treatment may be performed first, and composite restoration may be required for aesthetic reasons. Furthermore, it can be assumed that patients with multiple early caries enamel lesions may need bracket bonding after periodic remineralizing program. In this study, to explore the bonding properties of early carious enamel lesions after the application of remineralizing agents, an SBS test was performed on demineralized enamel coated with remineralizing agents. The results of this study showed that the SBS values after remineralization treatment were similar to those of the control group. There was no significant difference in the SBS values between the 24 h and 2 weeks groups.
In previous studies on the effects of remineralizing agents on the SBS of the enamel, the results varied. Abdelmegid et al. (5) reported that remineralizing agents (CPP-ACP and CPP-ACPF) applied to non-demineralized teeth decreased the bond strength because of the increased resistance to acid etching. Enan et al., (11) compared nHA and CPP-ACP for bonding orthodontic brackets and, reported that the mechanical properties improved as the enamel was remineralized, which had a positive effect on the bond strength. The discrepancies in these results might be dependent on various factors, including the degree of demineralization, remineralization, and etching performance in the bonding procedure.
The bond strength of adhesive materials is related to the mineral content of the tooth structure. After applying remineralizing agents, calcium and phosphate may have been deposited on the enamel surfaces. The presence of these surface layers may potentially hinder the action of the acid, reducing its ability to penetrate the enamel surface adequately. This could compromise the formation of resin tags, which are crucial for establishing sufficient micromechanical retention typically seen in enamel bonding (28). Therefore, remineralized enamel might not be readily etched and adhesive penetration is adversely affected. However, etching with 37% phosphoric acid prior to the application of the self-etching adhesive increased the bond strength. Inorganic content provided by the remineralizing agents was dissolved by the action of phosphoric acid-etching (29). Therefore, the effect of the remineralization agents may have been mitigated by phosphoric acid etching.
This study has some limitations. We performed only in the total-etch mode, considering that the usual clinical application of all bond universal on enamel is performed by the etch-and-rinse mode. However, it would have been better if the self-etch mode had also been used to compare the effect of the separated phosphoric etching step on the SBS to the remineralized enamel. Another limitation is that we designed the control group with only demineralized enamel; however, a negative control group with sound enamel would provide more detailed information regarding the comparison of bond strength. Therefore, pre-treatment with remineralization agents did not significantly affect the SBS when all bond universal was applied in the etch-and-rinse mode.
The Tooth Mousse Plus and Apapro used in this study are well-known to the public and can also be used as home care products. However, their effectiveness is not significantly superior to fluoride products. Therefore, the development of effective remineralizing agents for the multiple enamel demineralization lesions frequently encountered in orthodontic patients is a highly important issue. In this study, BGS-7 has been found to have a higher potential for remineralization compared to existing remineralizing agents, suggesting the possibility of developing more effective remineralizing agents using it. Therefore, further research under conditions more similar to the oral environment appears to be necessary.
Conclusions
Within the limitations of this study, BAG showed higher remineralization ability than all the other experimental agents. Notably, SBS was not affected by the remineralizing agents.
Acknowledgments
This paper was supported by Wonkwang University in 2023.
References
-
Yu OY, Lam WY, Wong AW, Duangthip D, Chu CH. Nonrestorative management of dental caries. Dent J (Basel). 2021 Oct;9(10):121.
[https://doi.org/10.3390/dj9100121]

-
Oliveira GM, Ritter AV, Heymann HO, Swift E Jr, Donovan T, Brock G, Wright T. Remineralization effect of CPP-ACP and fluoride for white spot lesions in vitro. J Dent. 2014 Dec;42(12):1592-602.
[https://doi.org/10.1016/j.jdent.2014.09.004]

-
Juntavee A, Juntavee N, Sinagpulo AN. Nanohydroxyapatite gel and its effects on remineralization of artificial carious lesions. Int J Dent. 2021 Nov; 2021:7256056.
[https://doi.org/10.1155/2021/7256056]

-
Ramadoss R, Padmanaban R, Subramanian B. Role of bioglass in enamel remineralization: Existing strategies and future prospects-A narrative review. J Biomed Mater Res B Appl Biomater. 2022 Jan;110(1):45-66.
[https://doi.org/10.1002/jbm.b.34904]

-
Abdelmegid FY, Salama FS, Abouobaid EI, Halawany HS, Alhadlaq MK. Effect of remineralizing agents on bond strength of resin-composites to primary enamel. J Clin Pediatr Dent. 2019;43(5):331-6.
[https://doi.org/10.17796/1053-4625-43.5.5]

-
Scribante A, Dermenaki Farahani MR, Marino G, Matera C, Rodriguez Y Baena R, Lanteri V, Butera A. Biomimetic effect of nano-hydroxyapatite in demineralized enamel before orthodontic bonding of brackets and attachments: Visual, adhesion strength, and hardness in in vitro tests. Biomed Res Int. 2020 Jan;2020:6747498.
[https://doi.org/10.1155/2020/6747498]

- Farias de Lacerda AJ, Ferreira Zanatta R, Crispim B, Borges AB, Gomes Torres CR, Tay FR, Pucci CR. Influence of de/remineralization of enamel on the tensile bond strength of etch-and-rinse and selfetching adhesives. Am J Dent. 2016 Oct;29(5):289-93.
-
Keçik D, Cehreli SB, Şar Ç, Ünver B. Effect of acidulated phosphate fluoride and casein phosphopeptide-amorphous calcium phosphate application on shear bond strength of orthodontic brackets. Angle Orthod. 2008 Jan;78(1):129-33.
[https://doi.org/10.2319/122506-529.1]

-
Bakry AS, Abbassy MA. The efficacy of a bioglass (45S5) paste temporary filling used to remineralize enamel surfaces prior to bonding procedures. J Dent. 2019 Jun;85:33-8.
[https://doi.org/10.1016/j.jdent.2019.04.010]

-
Xiaojun D, Jing L, Xuehua G, Hong R, Youcheng Y, Zhangyu G, Sun J. Effects of CPP-ACP paste on the shear bond strength of orthodontic brackets. Angle Orthod. 2009 Sep;79(5):945-50.
[https://doi.org/10.2319/101108-573.1]

-
Enan E, Tawfik MA, Mehesen R, Basha S. Remineralization potential and shear bond strength of surface treated hypomineralized enamel in bonding of orthodontic brackets: An in vitro Study. J Adv Oral Res. 2021 May;12(1):127-33.
[https://doi.org/10.1177/2320206820977734]

-
Abbassy MA, Bakry AS, Almoabady EH, Almusally SM, Hassan AH. Characterization of a novel enamel sealer for bioactive remineralization of white spot lesions. J Dent. 2021 Jun;109:103663.
[https://doi.org/10.1016/j.jdent.2021.103663]

-
Rana R, Itthagarun A, King NM. Effects of dentifrices on artificial caries like lesions: An in vitro pH cycling study. Int Dent J. 2007 Aug;57(4):243-8.
[https://doi.org/10.1111/j.1875-595X.2007.tb00127.x]

-
Farah RA, Swain MV, Drummond BK, Cook R, Atieh M. Mineral density of hypomineralised enamel. J Dent. 2010 Jan;38(1):50-8.
[https://doi.org/10.1016/j.jdent.2009.09.002]

-
Longbottom C, Ekstrand K, Zero D, Kambara M. Novel preventive treatment options. Monogr Oral Sci. 2009; 21:156-63.
[https://doi.org/10.1159/000224220]

-
Bakry AS, Takahashi H, Otsuki M, Tagami J. Evaluation of new treatment for incipient enamel demineralization using 45S5 bioglass. Dent Mater. 2014 Mar; 30(3):314-20.
[https://doi.org/10.1016/j.dental.2013.12.002]

-
Souza BM, Comar LP, Vertuan M, Fernandes Neto C, Buzalaf MA, Magalhães AC. Effect of an Experimental Paste with Hydroxyapatite Nanoparticles and Fluoride on Dental Demineralisation and Remineralisation in situ. Caries Res. 2015;49(5):499-507.
[https://doi.org/10.1159/000438466]

-
Somani R, Jaidka S, Singh DJ, Arora V. Remineralizing potential of various agents on dental erosion. J Oral Biol Craniofac Res. 2014 May-Aug;4(2):104-8.
[https://doi.org/10.1016/j.jobcr.2014.05.001]

-
Mehta AB, Kumari V, Jose R, Izadikhah V. Remineralization potential of bioactive glass and casein phosphopeptide-amorphous calcium phosphate on initial carious lesion: An in-vitro pH-cycling study. J Conserv Dent. 2014 Jan;17(1):3-7.
[https://doi.org/10.4103/0972-0707.124085]

-
Oh SH, Lee SR, Choi JY, Choi YS, Kim SH, Yoon HC, Nelson G. Detection of dental caries and cracks with quantitative light-induced fluorescence in comparison to radiographic and visual examination: A retrospective case study. Sensors (Basel). 2021 Mar; 21(5):1741.
[https://doi.org/10.3390/s21051741]

-
Cochrane NJ, Walker GD, Manton DJ, Reynolds EC. Comparison of quantitative light-induced fluorescence, digital photography and transverse microradiography for quantification of enamel remineralization. Aust Dent J. 2012 Sep;57(3):271-6.
[https://doi.org/10.1111/j.1834-7819.2012.01706.x]

-
Andersson ÖH, Kangasniemi I. Calcium phosphate formation at the surface of bioactive glass in vitro. J Biomed Mater Res. 1991 Aug;25(8):1019-30.
[https://doi.org/10.1002/jbm.820250808]

-
Burwell AK, Litkowski LJ, Greenspan DC. Calcium sodium phosphosilicate (NovaMin®): Remineralization potential. Adv Dent Res. 2009 Aug;21(1):35-9.
[https://doi.org/10.1177/0895937409335621]

-
Zhou C, Zhang D, Bai Y, Li S. Casein phosphopeptide-amorphous calcium phosphate remineralization of primary teeth early enamel lesions. J Dent. 2014 Jan;42(1):21-9.
[https://doi.org/10.1016/j.jdent.2013.11.005]

-
Mehta AB, Kumari V, Jose R, Izadikhah V. Remineralization potential of bioactive glass and casein phosphopeptide-amorphous calcium phosphate on initial carious lesion: An in-vitro pH-cycling study. J Conserv Dent. 2014 Jan;17(1):3-7.
[https://doi.org/10.4103/0972-0707.124085]

-
Elasser DM, Niazy MA, Elsharkawy DA, Mansour MS. The remineralizing potential of nano bioactive glass versus nanohydroxyapatite on dentine as affected by PH cycling, ADJ-for Girls. 2018 Oct;5(4):327-34.
[https://doi.org/10.21608/adjg.2018.20017]

-
Wang Z, Jiang T, Sauro S, Wang Y, Thompson I, Watson TF, Sa Y, Xing W, Shen Y, Haapasalo M. Dentine remineralization induced by two bioactive glasses developed for air abrasion purposes. J Dent. 2011 Nov;39(11):746-56.
[https://doi.org/10.1016/j.jdent.2011.08.006]

- Patel D, Sambrook R, Eder A. The effect of demineralization and remineralization cycles on the bond strength of resin composite to enamel. Eur J Prosthodont Restor Dent. 2021 May;29(2):93-101.
-
Elzuhery H, Fahmy OI, Elghandour IA, Ezzat MA, Abdalla AI. Bond strength and morphological interface of self-etching adhesives to demineralized and remineralized enamel. J Dent Sci. 2013 Sep;8(3):287-95.
[https://doi.org/10.1016/j.jds.2013.01.006]