Osteogenic potential of nerve growth factor incorporated gelatin methacryloyl beads: An in vitro study using MC3T3-E1
Abstract
Despite attempts to aid hard tissue regeneration using materials such as gelatin methacryloyl (GelMa), success has been limited. In this study, considering osteogenic differentiation and mineralization of MC3T3-E1 cells, nerve growth factor (NGF) was utilized for osteogenic differentiation by incorporating NGF into GelMA beads. GelMa beads with NGF (GBNGF) or without NGF (GB) were prepared. MC3T3-E1 cells were then encapsulated in GelMA beads fabricated by UV cross-linking. Live/Dead staining results showed that MC3T3-E1 cells in the GBNGF group exhibited more active cell-cell network formation and clearer spindle-shaped cell morphology than those in the GB group. Alizarin Red staining results revealed that MC3T3-E1 cells cultured in the GBNGF group had a higher amount of calcification indicated by a stronger red staining than those cultured in the GB group. Both results demonstrates that NGF could promote cell-cell and cell-matrix interactions, enhancing cell growth and osteogenic differentiation. In conclusion, the incorporation of NGF into GelMA beads can effectively promote growth and osteogenic differentiation of MC3T3-E1 cells. Therefore, GelMA beads containing NGF are expected to be utilized as a new therapeutic strategy for the regeneration of bone defects.
초록
젤라틴 메타크릴로일(GelMA)와 같은 물질을 사용하여 경조직 재생을 돕기 위한 시도에도 불구하고 성공은 제한적이었다. 본 연구에서는 MC3T3-E1세포의 골 형성 분화 및 광물화를 고려하여 신경성장인자(NGF)를 GelMA비드에 결합시켜 골 형성 분화에 신경성장인자를 활용하였다. NGF를 함유한 GelMA (GBNGF)와 NGF를 함유하지 않는 GelMA(GB) 를 제작하였고 MC3T3-E1 세포는 UV 가교에 의해 제작된 GelMA 비드에 캡슐화되었다. Live/Dead 염색 결과, GBNGF 그룹의 MC3T3-E1 세포는 GB 그룹보다 더 활발한 세포-세포 네트워크 형성과 명확한 방추형 세포 형태를 보였다. 또한, Alizarin Red 염색 결과, GBNGF 군에서 배양한 MC3T3-E1 세포는 GB 군에서 배양한 세포에 비해 더 강한 빨간색 염색으로 석회화량이 더 많은 것으로 나타났다. 두 결과 모두 NGF가 세포-세포 및 세포-기질 상호작용을 촉진하여 세포 성장과 골형성 분화를 향상시킬 수 있음을 보여준다. 결론적으로, GelMA 비드에 NGF를 결합시키면 MC3T3-E1 세포의 성장과 골 형성 분화를 효과적으로 촉진할 수 있다. 따라서, NGF를 함유한 GelMA 비드는 골결손 재생을 위한 새로운 치료 전략으로 활용될 수 있을 것으로 기대된다.
Keywords:
Gelatin methacryloyl (GelMA), Hydrogel, Nerve growth factor, 3D culture키워드:
젤라틴 메타크릴레이트, 하이드로겔, 신경성장인자, 3차원 배양Introduction
Bone has capacity to regenerate and repair itself. Thus, small bone defects can be regenerated naturally. However, in case of extensive defects or pathological fractures, surgical intervention such as bone grafting is required. In dentistry, bone defects can occur due to periodontal disease, trauma, and tumors (1). Dentists perform various types of surgery involving bone grafting to improve these oral bone defects (1, 2). Various types of biomaterials are used in bone grafting. Their main function is to provide mechanical support and promote bone regeneration. Healing of bone defect is a multifaceted process that includes osteoinduction, osteogenesis, and osteoconduction (3). Previous studies have reported that various growth factors such as transforming growth factor beta 1 (TGF-β1), platelet-derived growth factor-BB (PDGF-BB), and nerve growth factor (NGF) affect the differentiation and proliferation of MC3T3-E1 cell (4, 5). In particular, NGF can promote osteogenic capacity and the synthesis of vascular endothelial cell factor during fracture healing. It has also been found to be involved in bone formation in various processes such as skeletogenesis and ossification (6-8).
An ideal scaffold for bone tissue engineering (BTE) should be a suitable material with good biocompatibility that can transport cells or growth factors. Hydrogels contain a large amount of moisture with a porous structure similar to the extracellular matrix and an excellent biocompatibility. They could be used as materials to transport growth factors for promoting cell or bone formation (9). Because hydrogels provide a three-dimensional (3D) environment for cell growth and tissue formation, they can be widely used to observe cell-matrix, cell-cell interactions, proliferation, migration, and differentiation (10). 3D culture and two-dimensional (2D) culture environment show morphological and physiological differences. 3D culture has the advantage in that it enables us to observe results more similar to in vivo cellular responses than 2D culture (11).
Gelatin methacryloyl (GelMA) is a biopolymer-based hydrogel made by reacting gelatin and methacrylic anhydride. GelMA hydrogels are widely used in BTE because of their tunable mechanical properties, excellent photocrosslinking capability, and good biocompatibility (12). The reason why GelMA hydrogel has biocompatibility is because it contains arginine-glycin-aspartic acid (RGD) sequence related to cell adhesion and a peptide that is degraded by matrix metalloprotease (MMP). It can also be photocrosslinked because it contains a methacryloyl group (13). Due to this, GelMA has good moldability. It can take various shapes to suit the purpose (14). Many materials such as growth factors and inorganic nanoparticles can be encapsulated in GelMA along with cells to create a hybrid hydrogel. Therefore, in this study, we aimed to determine the effect of spherical GelMA beads containing NGF (GBNGF) on osteogenic differentiation. The null hypothesis is that the presence or absence of NGF in GelMA beads would have no significant effect on differentiation of MC3T3-E1.
Materials and Methods
1. Cell culture
MC3T3-E1 cells (CRL-2593; ATCC, Manassas, VA, USA) were cultured in minimum essential medium alpha modification (αMEM; Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and 1% antibiotic-antimycotic (AA; Gibco, Grand Island, NY, USA). These cells were maintained in a 37 ℃ incubator with 5% CO2. The culture medium was changed every 3 days after phosphate-buffered-saline (PBS, pH 7.4) washing and incubated at 37 ℃ with 5% CO2 atmosphere. These cultured cells were maintained under the same conditions as GelMA beads (GB) and GBNGF. They were used as a control group.
2. Preparation of GelMA solution and GelMA beads
GelMA (GeLMA 20%; CELLINK LLC, Boston, MA, USA), composed of gelatin methacrylate, PBS, and N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer solution, was dissolved in a mixture of PBS and 20 ng/mL NGF (11050-HNAE; Sino Biological Inc., Beijing, China) at a 1:1 ratio, respectively. The recombinant human NGF used in this study was lyophilized from sterile 0.01% trifluoroacetic acid (TFA) and consists of 40% acetonitrile, 5% trehalose, 5% mannitol, and 0.01% Tween-80. The mixture was then stirred at 65 ℃ for 10 minutes. 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959 or I2959; Sigma-Aldrich, St. Louis, USA) (1 wt%), a photoinitiator, was added and the mixture was further stirred at 65 ℃ for 10 minutes to prepare the GelMA solution (Table 1).
MC3T3-E1 cells were encapsulated in GelMA hydrogel at a concentration of 1×104 cells/mL. The mold used in this study was fabricated by pouring a mixed solution of deionized water (DW) and agarose (Inclonebiotech Co., Seongnam, Republic of Korea) into the tray of a casting mold (Cellrix® 3D Culture System; Cellrix, Seoul, Republic of Korea) and then, covering it with a 96-well cover of casting mold. The cell-containing GelMA solution (40 µL) was dispersed into each well of the mold and subsequently placed into a post-curing unit (LC-3D print Box; NextDent Co., Seosterberg, The Netherlands) with a wavelength band of 350~500 nm. The solution was cured for 30 seconds twice. GB and GBNGF were transferred into a 6-well plate and washed three times with PBS to remove unreacted photoinitiator (Figure 1).
3. Characterization of GelMA beads
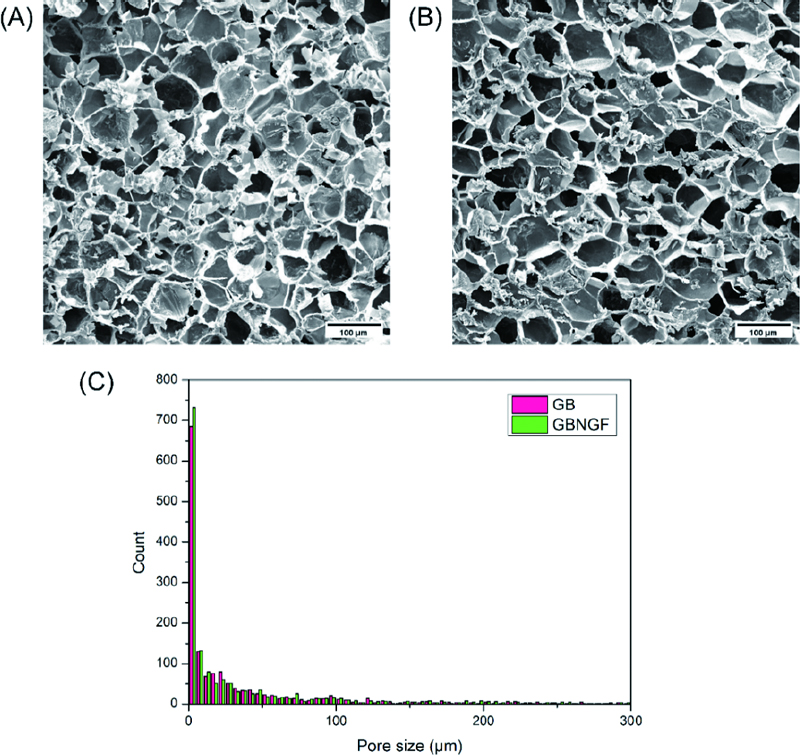
GB and GBNGF were frozen at −80 ℃, freeze dried overnight, sputter coated, and then imaged with a scanning electron microscope (SEM; Carl ZEISS, Oberkochen, Germany) for observation of porous structure. Pore sizes were compare quantitatively using ImageJ software (NIH, Bethesda, MD, USA).
4. Cell viability assessment using Live/Dead assay
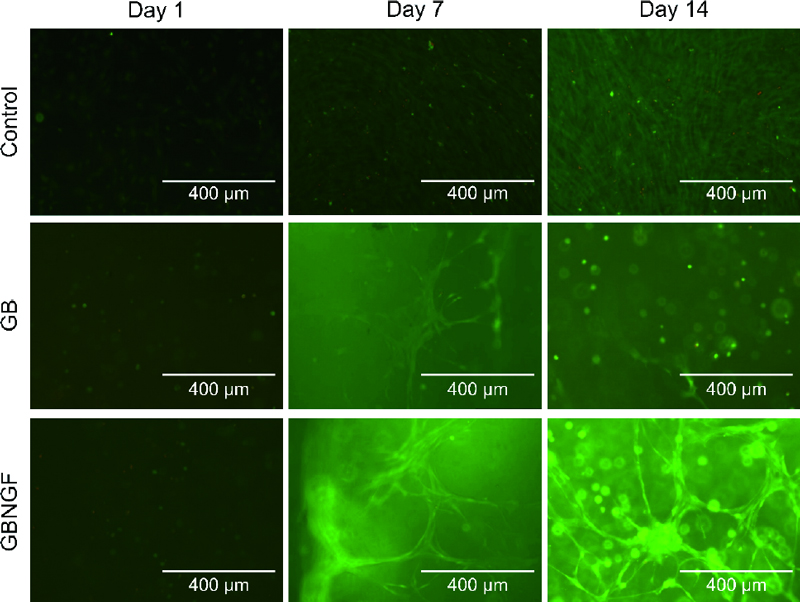
The viability of cells was analyzed using a Live/Dead Viability/Cytotoxicity Kit (Molecular Probes, Inc., Eugene, OR, USA). In brief, MC3T3-E1 cells in each group were incubated for 1, 7, and 14 days and washed with PBS. These cells were then stained with Live/Dead reagents for 30 min. Thereafter, stained cells were observed by confocal laser scanning microscopy (LSM 700, Zeiss, Oberkochen, Germany) to assess the viability of cells in each group.
5. Evaluation of osteogenic differentiation by Alizarin Red staining
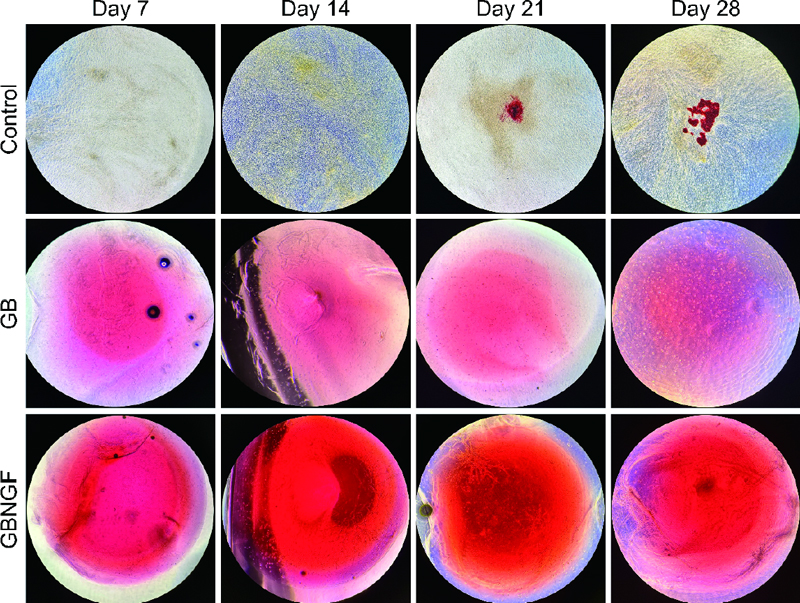
Alizarin Red staining (ARS) was performed to observe the formation of mineralized bone. After the culture medium was removed from each well, cells were gently washed three times with PBS. These cells were then fixed with 4% formaldehyde for 15 minutes at room temperature (RT). The formaldehyde was removed by washing three times with DW followed by adding 1 mL of 40 mM ARS solution (pH 4.2) into each well. Cells were incubated at RT for 30 seconds and ARS solutions were removed by suction. Cells were then washed with DW several times.
6. Statistical Analysis
Pore sizes of GB and GBNGF were analyzed with one-way analysis of variance (ANOVA) followed by Tukey’s statistical test using IBM SPSS Statistics version 27.0 (IBM Corp., Armonk, NY, USA).
Results
1. Characterization of GelMA beads
Figure 2(A) and Figure 2(B) show an irregular porous structure with small pores separated by thin walls present on both GB and GBNGF. Quantitative analysis using ImageJ showed that there were no significant (p > 0.05) differences in pore size between the two groups. The pore size distribution also showed similar patterns between the two groups (Figure 2(C)).
2. Cell viability and morphology
Live/Dead staining was performed to assess the viability of MC3T3-E1 cells cultured in different groups at day 1, day 7, and day 14 (Figure 3). As shown in the green fluorescence image, staining results revealed that most cells were alive throughout the culture period in all groups. No dead cells (red fluorescence) were detected at 1days. At days 7 and 14, increased cell density and cell-cell contacts were evident. Notably, cells in both GB and GBNGF groups appeared to be morphologically changed as MC3T3-E1 cells formed networks ranging from a round seed shape to an elongated spindle shape. Furthermore, the GBNGF group showed fewer dead cells (red fluorescence) than other groups.
3. Evaluation of mineralization and osteogenic differentiation
To evaluate osteogenic differentiation and mineralization of MC3T3-E1 cells, Alizarin Red staining was performed on days 7, 14, 21, and 28 for control, GB, and GBNGF groups (Figure 4). The red coloration indicating calcination was evident in all groups. It was more abundant in GB and GBNGF groups. Additionally, the GBNGF group had darker and more extensive staining than the GB group at all time points (7, 14, 21, and 28 days).
Discussion
There have been recent studies looking at osteogenic potential by adding various substances that can promote osteogenesis to hydrogels (15, 16). However, there is a lack of research about the effect of NGF on osteogenic potential in a 3D hydrogel environment. This study was conducted to investigate the effect of GelMA beads containing NGF called 3D hydrogels on bone differentiation.
The pore size and porous structure of hydrogels are important as they affect oxygen and nutrient diffusion and influence intercellular interactions (17-19). Some studies have reported that the presence of growth factor can affect porosity (20, 21). A previous study demonstrated that cells cultured in the presence of 20 ng/mL NGF promoted differentiation and neurite outgrowth in rat sympathetic nerves and brain cells, without inducing cell proliferation. Accordingly, this study also incorporated NGF at a concentration of 20 ng/mL into GelMA. SEM images in this study showed a porous structure with a mixture of large and small sized pores. There was no significant difference in the pore size of GelMA beads depending on whether or not they contained NGF. This appears to result from various factors associated with the GelMA bead fabrication process, including the concentration of incorporated growth factor, the GelMA content, and the degree of substitution.
The pore sizes of scaffolds for bone tissue growth were suggested to vary from 75 to 100 µm, 100 to 135 µm, and more than 300 µm (22, 23). The pore size diameter was approximately 10 to 100 µm, which is suitable for the attaching small particles and cells (24). In Figure 2, the pore sizes of GelMA were similar to those from previous studies on bone tissue growth.
GelMA in the form of 3D beads expands the surface area where cells can contact each other due to the curvature of the bead. It provides a favorable environment for cell growth by forming cell aggregation through cell proliferation and fusion. Additionally, the interconnected porosity inside beads can promote cell growth and uniform cell distribution. Due to these properties, 3D GelMA beads can activate intercellular communication and ultimately contribute to inducing differentiation (25, 26). LIve/Dead staining results indicated intercellular interactions within GelMA beads. In the control group, cells were grown densely. In the other group with a 3D structure, cells were grew connected in a straight line, creating a network. This result was consistent with previous research showing that when MC3T3-E1 cells were cultured in 3D in hydrogels, cells formed networks and changed to an elongated shape (27). Moreover, in the GBNGF group, larger and clearer spindle-shaped cells were observed after 7 and 14 days, suggesting that the presence of NGF could promote cell-cell and cell-matrix interactions.
ARS can be used to identify mineralized extracellular matrix because anthraquinone derivatives of ARS can react with Ca to form complexes (28). In late stages of osteoblast differentiation, calcium deposition leads to gradual calcification. Visual observation under a microscope showed increasing intensity of broad, dark red staining indicating progressive calcification in control, GB, and GBNGF groups, in that order. The increase in staining intensity indicates an increase in mineral deposition, suggesting that bone differentiation actively occurs (29). In particular, the intensity and range of staining were large in the GBNGF group, which could be seen as a result supporting that the differentiation in this group was the most active.
In this study, we confirmed that GBNGF could induce bone formation by promoting cell-cell and cell-matrix interactions without affecting pore size. However, because the optimal NGF concentration has not been identified, additional studies are needed to evaluate effects of various NGF concentrations on osteoblast differentiation.
Conclusions
This study suggests that incorporating NGF into GelMA beads can promote osteogenic differentiation of MC3T3-E1 cells. This approach is expected to be utilized as a new strategy for bone tissue regeneration.
Acknowledgments
This work was supported by the Technology Innovation Program (20009652, Technology on commercialization and materials of Bioabsorbable Hydroxyapatite that is less than micrometer in size) funded By the Ministry of Trade, Industry & Energy(MOTIE, Korea)
References
-
Jimi E, Hirata S, Osawa K, Terashita M, Kitamura C, Fukushima H. The current and future therapies of bone regeneration to repair bone defects. Int J Dent. 2012;2012(1):148261.
[https://doi.org/10.1155/2012/148261]

-
Ferraz MP. Bone grafts in dental medicine: an overview of autografts, allografts and synthetic materials. Materials. 2023 May 31;16(11):4117.
[https://doi.org/10.3390/ma16114117]

-
Zhou B, Jiang X, Zhou X, Tan W, Luo H, Lei S, et al. GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: therapeutic strategies and recent advances. Biomater Res. 2023; 27(1):86.
[https://doi.org/10.1186/s40824-023-00422-6]

-
Suzuki E, Ochiai-Shino H, Aoki H, Onodera S, Saito A, Saito A, et al. Akt activation is required for TGF-β1-induced osteoblast differentiation of MC3T3-E1 pre-osteoblasts. PLoS One. 2014;9(12):e112566.
[https://doi.org/10.1371/journal.pone.0112566]

-
Liu Q, Zhou Y, Li Z. PDGF‑BB promotes the differentiation and proliferation of MC3T3‑E1 cells through the Src/JAK2 signaling pathway. Mol Med Rep. 2018;18(4):3719-26.
[https://doi.org/10.3892/mmr.2018.9351]

-
Yang X, Mou D, Yu Q, Zhang J, Xiong Y, Zhang Z, et al. Nerve growth factor promotes osteogenic differentiation of MC3T3-E1 cells via BMP-2/Smads pathway. Ann Anat. 2022;239:151819.
[https://doi.org/10.1016/j.aanat.2021.151819]

-
Yada M, Yamaguchi K, Tsuji T. NGF Stimulates Differentiation of Osteoblastic MC3T3-E1 Cells. Biochem. Biophys. Res. Commun. 1994;205(2):1187-93.
[https://doi.org/10.1006/bbrc.1994.2791]

-
Jadlowiec J, Koch H, Zhang X, Campbell PG, Seyedain M, Sfeir C. Phosphophoryn regulates the gene expression and differentiation of NIH3T3, MC3T3-E1, and human mesenchymal stem cells via the integrin/ MAPK signaling pathway. J Biol Chem. 2004;279(51):53323-30.
[https://doi.org/10.1074/jbc.M404934200]

-
Yue S, He H, Li B, Hou T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials (Basel). 2020;10(8):1511
[https://doi.org/10.3390/nano10081511]

-
Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. J Biomater. 2015;73:254-71.
[https://doi.org/10.1016/j.biomaterials.2015.08.045]

-
Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12(4):207-18.
[https://doi.org/10.1089/adt.2014.573]

-
Zhou B, Jiang X, Zhou X, Tan W, Luo H, Lei S, et al. GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: therapeutic strategies and recent advances. Biomater Res. 2023;27(1):86.
[https://doi.org/10.1186/s40824-023-00422-6]

-
Bupphathong S, Quiroz C, Huang W, Chung P-F, Tao H-Y, Lin C-H. Gelatin Methacrylate Hydrogel for Tissue Engineering Applications—A Review on Material Modifications. Pharmaceuticals (Basel). 2022;15(2):171.
[https://doi.org/10.3390/ph15020171]

-
Piao Y, You H, Xu T, Bei H-P, Piwko IZ, Kwan YY, et al. Biomedical applications of gelatin methacryloyl hydrogels. Eng Regen. 2021;2:47-56.
[https://doi.org/10.1016/j.engreg.2021.03.002]

-
Wang Y, Ma M, Wang J, Zhang W, Lu W, Gao Y, et al. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials (Basel). 2018;11(8).
[https://doi.org/10.3390/ma11081345]

-
Thakur T, Xavier JR, Cross L, Jaiswal MK, Mondragon E, Kaunas R, et al. Photocrosslinkable and elastomeric hydrogels for bone regeneration. J Biomed Mater Res A. 2016;104(4):879-88.
[https://doi.org/10.1002/jbm.a.35621]

-
Annabi N, Nichol JW, Zhong X, Ji C, Koshy S, Khademhosseini A, et al. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng Part B Rev. 2010;16(4):371-83.
[https://doi.org/10.1089/ten.teb.2009.0639]

-
Huang X, Lou Y, Duan Y, Liu H, Tian J, Shen Y, et al. Biomaterial scaffolds in maxillofacial bone tissue engineering: A review of recent advances. Bioact Mater. 2024;33:129-56.
[https://doi.org/10.1016/j.bioactmat.2023.10.031]

-
Pepelanova I, Kruppa K, Scheper T, Lavrentieva A. Gelatin-Methacryloyl (GelMA) Hydrogels with Defined Degree of Functionalization as a Versatile Toolkit for 3D Cell Culture and Extrusion Bioprinting. Bioengineering (Basel). 2018 Jul 18;5(3):55.
[https://doi.org/10.3390/bioengineering5030055]

-
An J, Shi X, Zhang J, Qi L, Xue W, Nie X, et al. Dual aldehyde cross-linked hyaluronic acid hydrogels loaded with PRP and NGF biofunctionalized PEEK interfaces to enhance osteogenesis and vascularization. Mater Today Bio. 2024;24:100928.
[https://doi.org/10.1016/j.mtbio.2023.100928]

-
Luo L, He Y, Jin L, Zhang Y, Guastaldi FP, Albashari AA, et al. Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries. Bioact Mater. 2021;6(3):638-54.
[https://doi.org/10.1016/j.bioactmat.2020.08.028]

-
Murphy CM, O'Brien FJ. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh Migr. 2010;4(3):377-81.
[https://doi.org/10.4161/cam.4.3.11747]

-
Pishavar E, Luo H, Naserifar M, Hashemi M, Toosi S, Atala A, et al. Advanced Hydrogels as Exosome Delivery Systems for Osteogenic Differentiation of MSCs: Application in Bone Regeneration. Int J Mol Sci. 2021 Jun 8;22(12):6203.
[https://doi.org/10.3390/ijms22126203]

-
Shen C, Zhao X, Ren Z, Yang B, Wang X, Hu A, et al. In Situ Formation of Injectable Gelatin Methacryloyl (GelMA) Hydrogels for Effective Intraocular Delivery of Triamcinolone Acetonide. Int J Mol Sci. 2023; 24(5):4957.
[https://doi.org/10.3390/ijms24054957]

-
Jiang J, Liu A, Chen C, Tang J, Fan H, Sun J, et al. An efficient two-step preparation of photocrosslinked gelatin microspheres as cell carriers to support MC3T3-E1 cells osteogenic performance. Colloids Surf B Biointerfaces. 2020;188:110798.
[https://doi.org/10.1016/j.colsurfb.2020.110798]

-
El-Sherbiny IM, Yacoub MH. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob Cardiol Sci Pract. 2013;2013(3):316-42.
[https://doi.org/10.5339/gcsp.2013.38]

-
Marí-Buyé N, Luque T, Navajas D, Semino CE. Development of a three-dimensional bone-like construct in a soft self-assembling peptide matrix. Tissue Eng Part A. 2013;19(7-8):870-81.
[https://doi.org/10.1089/ten.tea.2012.0077]

-
Bernar A, Gebetsberger JV, Bauer M, Streif W, Schirmer M. Optimization of the Alizarin Red S Assay by Enhancing Mineralization of Osteoblasts. Int J Mol Sci. 2023;24(1):723.
[https://doi.org/10.3390/ijms24010723]

-
Jeon J, Lee MS, Yang HS. Differentiated osteoblasts derived decellularized extracellular matrix to promote osteogenic differentiation. Biomater Res. 2018;22(1):4.
[https://doi.org/10.1186/s40824-018-0115-0]