Anti-inflammation effect of ethanol extracts composed of propolis, pineapples, sea buckthorn fruit, figs, and kiwi extracts on Raw 264.7 cells treated with lipopolysaccharide
Abstract
The purpose of this study was to investigate anti-inflammatory effects of a mixture of ethanol extracts of propolis, pineapple, sea buckthorn fruit, fig, and kiwi on Escherichia coli-derived lipopolysaccharide (LPS)-stimulated Raw 264.7 cells. Antioxidant activity of the mixture of ethanol extracts of propolis, pineapples, hawthorn fruits, figs, and kiwis were determined by ABTS (2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)) assay. Nitric oxide (NO) production measured via the Griess method confirmed an anti-inflammatory effect of the extract mixture. The expression levels of tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), and interleukin-6 (IL-6) were determined by reverse transcription polymerase chain reaction (RT-PCR). The highest antioxidant activity was observed for extract mixture No. 4 (propolis:pineapple:sea buckthorn fruit:fig:kiwi at 3:3:2:1:1). ABTS assays revealed that treatment with the extract mixture No. 4 at 100 μg/mL resulted 52.3% free radical scavenging ability, demonstrating a higher efficacy of the mixture than single-extract treatments (p>0.5). The mixture of five extracts effectively inhibited the production of nitrogen compounds. Treatment with extract mixture No. 4 at a concentration of 100 μg/mL resulted in a 25.6% reduction in NO production compared to LPS treatment alone. Analysis of cytokine expression patterns during an inflammatory response revealed that expression levels of TNF-α, IL-1β, and IL-6 were decreased by the extract mixture in a concentration-dependent manner. These results suggest that the extract mixture No. 4 might be useful for developing preventive or therapeutic products targeting various inflammatory diseases, including periodontal disease.
초록
이 연구는 프로폴리스, 파인애플, 산자나무 열매, 무화과, 키위의 에탄올 추출 혼합물이 대장균 유래 지질다당류로 자극된 Raw 264.7 세포에 미치는 항염증 효과를 확인고자 진행되었다. 프로폴리스, 파인애플, 산자나무 열매, 무화과, 키위의 에탄올 추출물 혼합물들의 항산화능은 ABTS [2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)] 분석법으로 측정하였다. Griess 방법을 통해 측정된 일산화질소(NO) 생성은 추출물 혼합물의 항염증 효과를 확인하였다. 종양괴사인자-α(TNF-α), 인터루킨 1β(IL-1β), 그리고 인터루킨-6(IL-6)의 발현 수준은 역전사 중합효소 연쇄반응(RT-PCR)에 의해 결정하였다. 추출물 혼합물 No. 4 (프로폴리스:파인애플:산자나무 열매:무화과: 키위의 비율이 3:3:2:1:1)에서 가장 높은 항산화 활성이 나타나는 것을 확인하였다. ABTS 분석 결과, 100 μg/mL 농도로 처리된 추출물 혼합물 No. 4는 52.3%의 free radical 소거능을 나타내어 혼합조건에서 가장 높은 활성을 보여주었다 (p>0.5). 항염증 효과를 확인하기 위하여 Griess 방법을 통해 산화질소 화합물(NO) 생산량을 확인하였다. 추출물 혼합물 No. 4 100 μg/mL 농도로 처리하였을 경우, LPS 단독 처리 시보다 NO 생성량이 25.6% 감소하였다. 염증 반응 유도 시 염증 유발 사이토카인 발현 양상을 확인한 결과, TNF-α, IL-1β 및 IL-6 발현 양상이 추출물 혼합물에 의해 농도 의존적으로 감소되는 것을 확인하였다. 이러한 결과는 추출물 혼합물 No. 4가 다양한 염증성 질환, 특히 치주 질환을 대상으로 하는 예방 또는 치료 제품 개발에 사용될 수 있을 것으로 판단된다.
Keywords:
Antioxidant, Anti-inflammatory agent, Lipopolysaccharide, Cytokines, RAW 264.7 Cell line키워드:
항산화제, 항염증제, 지질다당류, 사이토카인, RAW 264.7 세포주Introduction
Periodontitis is a common inflammatory disease that can lead to tooth loss due to destruction of the tissue supporting the teeth. It been linked to a variety of systemic diseases, including cardiovascular disease and diabetes (1, 2). Oxidative stress is caused by reactive radicals called free radicals known to be involved in immune responses and intracellular signaling. These free radicals play a key role in the progression of many inflammatory diseases. There is a growing body of research linking oxidative stress to the development of inflammatory diseases. The inflammatory response is one of the body’s defense mechanisms against bacterial infections, chemicals, and external stimuli (3, 4). Various stimuli can lead to the production of inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), and interleukin-6 (IL-6). Excessive or recurrent production of these cytokines may contribute to the development of various diseases, including tumors and chronic inflammatory diseases. Thus, the search for anti-inflammatory substances that can modulate the expression or activity of these inflammatory cytokines may help prevent or ameliorate diseases (5-7).
Natural products have been used as teas, foods, and folk remedies since ancient times. Some of them have been used as medicines. In addition, natural products are often used in perfumes, food supplements, and dietary supplements due to their low side effects (4-6, 8). Propolis is a mixture of substances produced by bees to defend their hive, seal holes and cracks, and rebuild the hive. Propolis and propolis extracts are known for their antioxidant activities and antimicrobial-related properties. They have been widely studied for applications as antiseptic, anti-inflammatory, antioxidant, antibacterial, antifungal, and immunomodulatory agents (8-10). The main active substance in pineapple is bromelain known to have a variety of proteolytic, anti-edematous, antithrombotic, and anti-inflammatory properties (7, 11). Hippophae rhamnoides L. is also known as the sea buckthorn or vitamin tree. Its fruits and seeds are known to contain vitamins, carotenoids, plant sterols, minerals, organic acids, and polyunsaturated fatty acids, which are used in food, cosmetics, and medicine (5, 6). Figs contain vitamins, minerals, carbohydrates, organic acids, and phenolic compounds. They are particularly high in fiber and polyphenols. Its fruits, roots, and leaves have been used to treat gastrointestinal, respiratory, cardiovascular, inflammatory and convulsant diseases (12, 13). Kiwi is a fruit eaten in many parts of the world because it contains a variety of bioactive substances including vitamins C, E, and K, dietary fiber, potassium, and folate. Particularly, it contains actinidine, an active enzyme that can break down proteins and facilitate digestion in the stomach and intestines (14, 15). Natural products are favorable for manufacture or use as components of health functional foods and cosmetics because they have few side effects and drug resistance problems (4, 16). There are many studies on the efficacy of individual extracts, but there is a lack of research on whether combining several physiologically active natural products results in synergistic effects and various physiological activities. The aim of this study was to determine anti-inflammatory effects of a mixture of ethanol extracts of propolis, pineapple, sea buckthorn fruit, fig, and kiwi on Escherichia coli-derived lipopolysaccharide (LPS)-stimulated Raw 264.7 cells.
Materials and Methods
1. Extracting natural products
Propolis (Australia), pineapple (Philippines), sea buckthorn fruit (China), fig (Republic of Korea), and kiwi (New Zealand) were washed, cut into 3-5 cm pieces, and placed in 10 parts of 70% ethanol. After extracting at 30~40 ℃ under shaded conditions for one week, the crude product was filtered through a paper filter (5 μm, Advantec, Uchisaiwaicho, Tokyo), Concentration was carried out using an evaporator (IKA, Staufe, Germany) at 42–45 ℃ and 100–200 rpm, followed by freeze-drying for use (Figure 1). Each lyophilized extract was dissolved in 10% ethanol to a concentration of 200 mg/mL. A mixture of the five extracts (Table 1) was used for the following experiments.
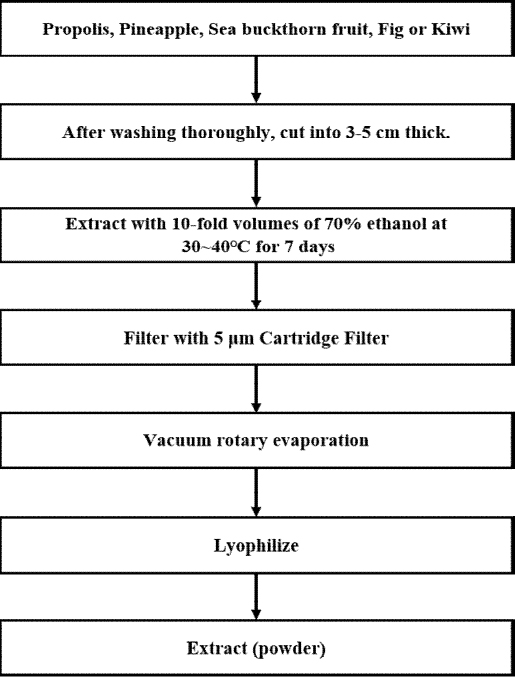
Schematic representation of the ethanol extraction process for propolis, pineapple, sea buckthorn fruit, fig, and kiwi.
2. Cytotoxicity measurements
Raw 264.7 cells used for cytotoxicity experiments were purchased from Korean Cell Line Bank (Korean Cell Line Bank). They were cultured in DMEM supplemented with 10% fetal bovine serum (ATLAS, Fort Collins, CO, USA), 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA) and incubated at 37 ℃ with 5% CO2. Fully cultured Raw 264.7 cells were seeded into 96-well plates at a density of 2×104 cells/well and incubated for 24 h. After the incubation, solutions of the mixture of five ethanol extracts with 12 different combinations were added to the plate (final concentration: 25, 50, 100, 200 μg/mL) (7, 12, 19, 24) and cells were incubated for 24 h at 37 ℃ with 5% CO2. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) (Sigma-Aldrich, St. Louis, MO, USA) was then added to each well to determine cell viability. After 10 μL of MTT solution (5 mg/mL) was added to each well, plates were further incubated at 37 ℃ with 5% CO2 for 4 h. The negative control was a solution of 0.5% ethanol in cell culture medium. After further incubation, the supernatant was carefully removed and 200 μL of dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA) was added to each well followed by incubation with shaking room temperature at 100 rpm for 30 min. Absorbance was then measured at 540 nm using a microplate reader (Epoch, Biotek, Winooski, VT, USA). Cell viability was calculated with the following formula (1, 4):
Cell viability (%) = (1 - sample treated group Optical Density (OD)/control group OD)×100
3. Radical scavenging capacity measurement
Antioxidant capacity of the extract was determined by ABTS (2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)) radical scavenging activity assay. An ABTS standard solution was prepared by mixing 7 mM ABTS (Sigma-Aldrich, St. Louis, MO, USA) with 2.45 mM potassium persulfate (Sigma-Aldrich, St. Louis, MO, USA) and allowed to stand for 24 h at room temperature before use. The ABTS standard solution and the mixture of five ethanol extracts (0.1 mL each) were added to a 96-well plate and incubated at room temperature in the dark for 20 min. The absorbance at 734 nm using a microplate reader (Epoch, Biotek, Winooski, VT, USA). The radical scavenging activity was calculated with the following formula (4, 8):
Free Radical scavenging activity = ((Blank OD - sample treat OD)/(Blank OD))×100
4. Nitric oxide (NO) production assay
NO production was measured using the Griess reagent (Sigma-Aldrich). Raw 264.7 cells were plated at 1×105 cells/plate in 24-well plates after sufficient culture, and then treated with 500 ng/mL of LPS (Escherichia coli, O111:B4, Sigma-Aldrich) and five extraction mixtures (Table 1) at different concentrations (25, 50, 100 μg/mL) and incubated for another 24 h. 100 μL of the incubated medium supernatant was taken into a 96 well plate, and an equal amount of Griess reagent was added to it, reacted for 10 min, and the absorbance was measured at 542 nm using a microplate reader (Epoch, Biotek, Winooski, VT, USA) (3, 7).
5. Measurement of inflammation-related mRNA expression by PCR
Raw 264.7 cells were cultured in 6-well plates at 3× 105 cells/plate, then treated with LPS (Sigma-Aldrich, St. Louis, MO, USA) 500 ng/mL and five extraction mixtures (Table 1) at different concentrations (25, 50, 100 μg/mL) and incubated for another 3 h. The medium supernatant was removed, washed thoroughly with PBS, and RNA was extracted using an RNA Extraction Kit (Bioneer, Daejeon, Korea). RNA was quantified using a Nanodrop (Thermo Fisher, Waltham, MA, USA). cDNA was synthesized using RT Premix kit (Bioneer) by reacting at 25 ℃ for 5 min, 42 ℃ for 60 min, and 72 ℃ for 15 min.
Anti-inflammatory primers were prepared and used as shown in Table 2, and PCR was performed using PCR premix (Bioneer), followed by electrophoresis on a 1% agarose gel to check for changes in expression.
6. Identification of inflammatory cytokine factors in cell cultures
To identify the inflammatory cytokines secreted by Raw 264.7 cells, TNF-α, IL-1β, and IL-6 (RayBiotech, Norcross, GA, USA), an enzyme-linked immunosorbent assay (ELISA) kit was used. Raw 264.7 cells were cultured in 12-well plates at 2×105 cells/plate and then treated with LPS (Sigma-Aldrich, St. Louis, MO, USA) 500 ng/mL and five extraction mixtures (Table 1) at different concentrations (25, 50, 100 μg/mL) and incubated for another 24 h. The supernatants were collected, centrifuged and measured using the respective ELISA kits.
Results
1. Cytotoxicity of the extracts at different mixing ratios
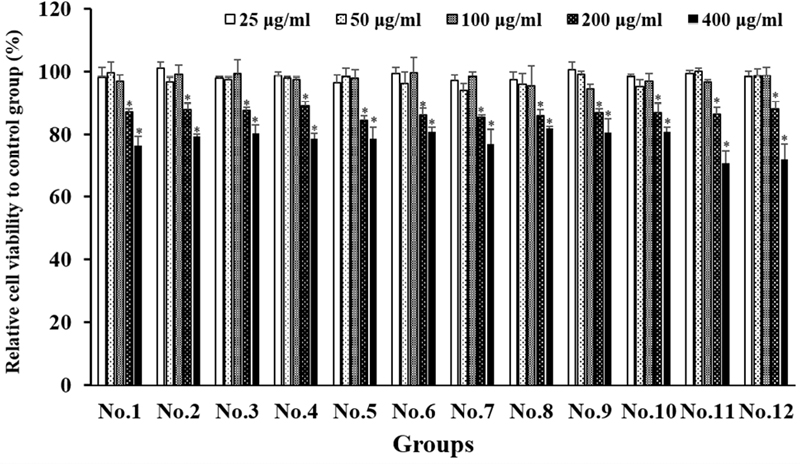
The cytotoxicity of the extracts was evaluated after treatment (Figure 2). No cell morphology changes, or cytotoxicity was observed at all mixing ratios or alone of the concentration until 100 μg/mL treated, and a slight increase in cytotoxicity was observed at the concentration of 200 μg/mL. Further experiments were performed up to 100 μg/mL, the maximum concentration at which no cytotoxicity was seen.
2. Radical scavenging capacity as a function of extract mixture ratio
The antioxidant activity was measured using the ABTS method to determine the radical scavenging capacity. In the case of single extracts, propolis, pineapple, and vitamin tree fruit extracts showed high radical scavenging capacity and were compared together. The ABTS assay was used to measure the activity of the extracts in different mixtures (Figure 3), and the highest activity was observed in extract mixture No. 3 and No. 4. They showed higher radical scavenging capacity than the No. 8, No. 9, No. 10, No. 11 and No. 12 extracts treated alone (Figure 3). While the positive control showed lower radical scavenging capacity than butylated hydroxyanisole (BHA), extract mixture No. 4 (propolis:pineapple:sea buckthorn fruit:fig:kiwi=3:3:2:1:1:1) showed 30% radical scavenging capacity at 25 μg/mL and 52.3% radical scavenging capacity at 100 μg/mL. The radical scavenging capacity of extract mixture No. 4 was higher than that of extract mixture No. 8 (propolis), which showed a radical scavenging capacity of 48.9% at a concentration of 100 μg/mL.
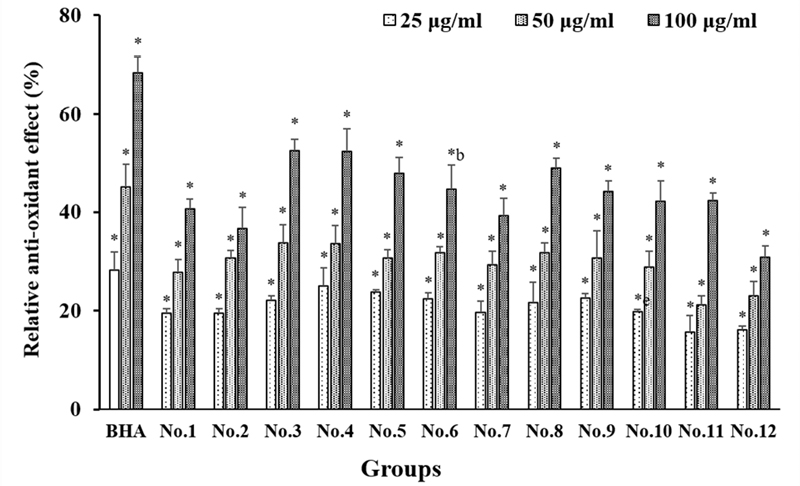
Radical scavenging activities of extract mixtures assessed by ABTS assay. Extract concentrations were 25, 50, and 100 μg/mL. Free radical scavenging capacity was determined using ABTS assay. Data are presented as means ± SD (n = 3). *, Significantly different compared to dissolved solvent (0.5% EtOH) at p<0.001.
3. Effect of extract mixing conditions on inhibition of nitric oxide production
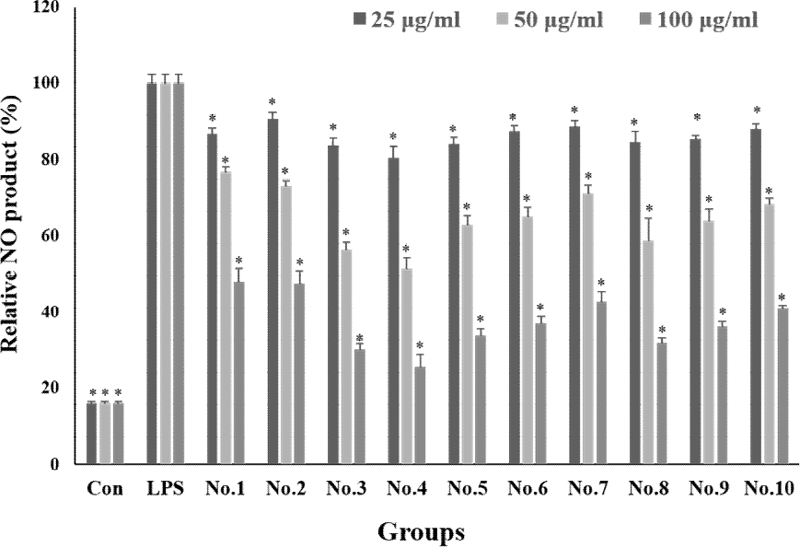
Nitric oxide (NO) production was expressed as a percentage based on the Griess assay results of the LPS treated group (Figure 4). It was found that the production of NO induced by the inflammatory response by LPS treatment was reduced in a concentration-dependent manner when the extraction mixture was treated. In the untreated group, the level of NO was around 15%. In the single treatment groups, at a concentration of 100 μg/mL, propolis extract (No. 8) reduced nitrogenous compounds by 31.7%, pineapple extract (No. 9) by 36.2%, and vitamin tree fruit extract (No. 10) by 40.9%. When the extracts were treated by mixing them, at a concentration of 100 μg/mL, the extract mixture No. 3 was 30.2%, the extract mixture No. 4 was 25.6%, an.d the extract mixture No. 5 was 33.8%, indicating a decrease in the nitrogen compound of about 67 to 74%. Comparing the propolis extract, which was the most active of the single treatment groups, with the fourth mixture ratio, there was a difference in activity of about 6%. Although the difference was not significant, we found that the activity was slightly higher in the mixed treatment.
4. Suppression of gene expression of inflammation-related cytokines
The expression of inflammatory cytokines, TNF-α, IL-1 β, and IL-6, was checked by PCR. When R aw264.7 cells were treated with LPS alone to induce an inflammatory response, we found that the expression levels of TNF-α, IL-1β, and IL-6 were increased. However, when Raw 264.7 cells were treated with LPS and extract mixture No. 4 at concentrations of 25~100 μg/mL to check the expression of inflammatory cytokines, we found that the expression levels of TNF-α, IL-1β, and IL-6 were decreased in a concentration-dependent manner (Figure 5A). When extract mixture No. 4 was treated with 100 μg/mL, the density of PCR bands was measured by image J program, and it was found that the expression level of TNF-α was reduced by 38%, IL-1β by 56%, and IL-6 by 31% compared to that of LPS alone.
5. Inhibitory effect on secretion of inflammation-related cytokines
Changes in secreted cytokines an inflammatory response induced by LPS in raw 264.7 cells after treatment with extract mixture No. 4 were then determined. When Raw 264.7 cells were treated with LPS, secretion levels of TNF-α and IL-1β were increased. However, after treatment with extract mixture No. 4 at 100 μg/mL, secretion levels of TNF-α and IL-1β were reduced by about 70% compared to the control (LPS treatment alone). The amount of secretion of IL-6 was reduced by about 50% after treatment with extract mixture No. 4 at 100 μg/mL (Figure 5B).
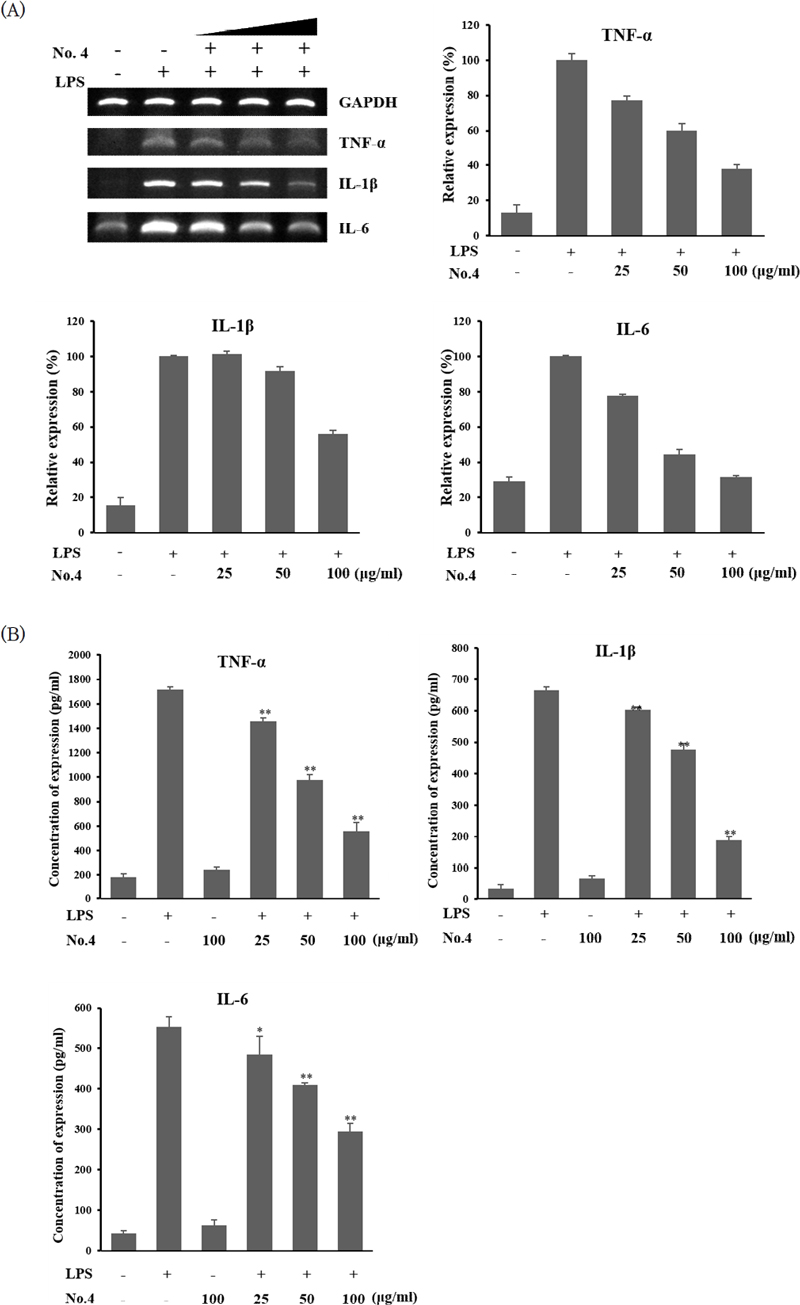
Effect of extract mixture No. 4 on cytokine expression in LPS-induced Raw 264.7 cells. (A) mRNA expression levels of inflammatory cytokines as detected by PCR. (B) Secreted cytokine levels in culture medium quantified by ELISA. Extract concentrations were 25, 50, and 100 μg/mL. Data are presented as means ± SD (n = 3). *, p<0.05 compared to LPS-only treatment; **, p<0.001 compared to LPS-only treatment.
Discussion
In this study, raw 264.7 cells with LPS-induced inflammation were treated with ethanolic extract of each natural product alone or in combination of them to determine the optimal mixing ratio of five natural extracts that exhibited the highest anti-inflammatory and antioxidant activities.
Results of this study showed that extract mixture No. 4 and No. 3 showed the highest biological activities. Reactive oxygen species (ROS) such as hydrogen peroxide and hydroxyl radicals, which are byproducts of various reactions and physical activities in the human body, exhibit a wide range of toxicities in vivo. They might be responsible for many oral inflammation symptoms (1, 2, 17). Among many natural products, propolis, pineapple, sea buckthorn fruit, figs, and kiwi have been reported to have antioxidant activities at concentrations of 20-100 μg/mL (8, 11, 12, 14, 18). In this study, results were similar (about 30-80% activities at 25-100 μg/mL). Extract mixture No. 4, a mixture of propolis, pineapple, sea buckthorn fruit, fig, and kiwi ethanol extracts at a ratio of 3:3:2:1:1 showed the highest activity of 52.3% at 100 μg/mL in ABTS assay. This was about 3.4% higher than the propolis extract alone (No. 8). These results indicate that the extract mixture No. 4 has a very high antioxidant capacity. At a concentration of 100 μg/mL, no cytotoxicity was detected, confirming that its antioxidant activity was sufficient.
In this study, raw 264.7 cells induced by LPS were treated with ethanol extracts of five natural products alone or in combination to measure the amount of nitric oxide generated during inflammation to confirm their antiinflammatory activities. Results showed a similar trend to previous antioxidant activity measurement results (i.e., about 75% inhibition of nitric oxide production by extract mixture No. 4, which was about 6% more than that of propolis extract at 69%). Cytokines are inflammatory factors that appear during inflammatory responses. Their expression pattern was checked with PCR and ELISA kits. Results showed that gene expression levels of inflammatory cytokines such as TNF-α, IL-1β, and IL-6 were increased upon LPS treatment. However, their expression levels were decreased after treatment with extract mixture No. 4. We also found that levels of secreted cytokines were inhibited by extract mixture No. 4. These results are similar to previous studies (5-7, 9) showing that propolis, pineapple, and sea buckthorn fruit extracts can inhibit nitric oxide production, thereby suppressing the production of nitric oxide and inflammatory cytokines that appear during inflammatory responses. Taken together, these results suggest that extract mixture No. 4 has both antioxidant and anti-inflammatory properties.
Propolis is already used in many anti-inflammatory products because it contains active ingredients such as pinocembrin, cinnamic acid, and apigenin known to have excellent antioxidant and antimicrobial activities (8, 9, 19, 20). Pineapple contains a proteolytic enzyme called bromelain known to possess antibacterial, antioxidant, and anti-inflammatory activities. It has been used therapeutically since ancient times (7, 11). Sea buckthorn fruit extracts contain vitamins B and C for their antioxidant and anti-inflammatory activities (21, 22). Figs are cultivated in many countries. They have been used as medicinal plants for their active ingredients including ficin, vitamins, minerals, and various polyphenols (5, 13, 23). Kiwi contains various active substances such as actinidin, vitamins C, K, and E known to have antioxidant and anti-inflammatory activities (18, 24, 25). These materials utilized in this study are easily accessible in our daily lives. They have no side effects even if they are used for a long period of time. They are easy to access as materials with little rejection when incorporated into products.
To summarize results of this study, a mixture of propolis, pineapple, sea buckthorn fruit, fig, and kiwi ethanol extracts at a ratio of 3:3:2:1:1 was not cytotoxic as well as excellent antioxidant and anti-inflammatory functions. Thus, it has potential to be used in the development of preventive or therapeutic products targeting various inflammatory diseases including periodontal disease.
Conflict of interest
H.J.C and C.K.L are co-register on the patent (No.: 10-2630716, Korea) entitled “Composition for oral hygiene.” The other author has no conflict of interest.
References
-
Kim YH, Park BS. Antioxidant Effect of Eugenol in Human Periodontal Ligament Fibroblasts. Korean J Phys Anthropol. 2015;28(1):45-53.
[https://doi.org/10.11637/kjpa.2015.28.1.45]

-
Wang Y, Andrukhov O, Rausch-Fan X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017;8:910.
[https://doi.org/10.3389/fphys.2017.00910]

-
Baek SH, Park T, Kang MG, Park DU. Effect of Sinapaldehyde in LPS-Stimulated RAW 264.7 Macrophages. Molecules. 2020;25(18):4089.
[https://doi.org/10.3390/molecules25184089]

- Lee JH. Antioxidant, Antibacterial and Anti-inflammatory Effects of Stachys sieboldii Extract. Korean J. Plant Res. 2021;34(5):420-32.
-
Baek SC, Lee DH, Jo MS, Lee KH, Lee YH, Kang KS, Yamabe N, Kim KH. Inhibitory Effect of 1,5-Dimethyl Citrate from Sea Buckthorn (Hippophae rhamnoides) on Lipopolysaccharide-Induced Inflammatory Response in RAW 264.7 Mouse Macrophages. Foods. 2020;9(3):269.
[https://doi.org/10.3390/foods9030269]

-
Han Y, Yuan C, Zhou X, Han YJ, He YH, Ouyang JA, Zhou Wn, Wang ZH, Wang HL, Li G. Anti-Inflammatory Activity of Three Triterpene from Hippophae rhamnoides L. in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Int J Mol Sci. 2021;22(21):12009.
[https://doi.org/10.3390/ijms222112009]

-
Insuan O, Janchai P, Thongchuai B, Chaiwongsa R, Khamchun S, Saoin S, Insuan W, Pothacharoen P, Apiwatanapiwat W, Boondaeng A, Vaithanomsat P. Anti-Inflammatory Effect of Pineapple Rhizome Bromelain through Downregulation of the NF-κB- and MAPKs-Signaling Pathways in Lipopolysaccharide (LPS)-Stimulated RAW 264.7 Cells. Curr Issues Mol Biol. 2021;43(1):93–106.
[https://doi.org/10.3390/cimb43010008]

-
Jeong CH, Shin CS, Bae YI, Shm KH. Antioxidant Activities of Ethanol and Water Extracts from Propolis. J Korean Soc Food Sci Nutr. 2010;39(12):1725-30.
[https://doi.org/10.3746/jkfn.2010.39.12.1725]

- Lee JW. Analysis of Anti-inflammatory Effect on Odorless Korea Propolis. Journal of Knowledge Information Technology and Systems. 2020;15(2):275-83.
-
Sung SJ, Kang KM, Lee KH, Yoo SY, Kook JK, Lee DS, Yu SJ. Effect of Garcinia mangostana L. and propolis extracts on the inhibition of inflammation and alveolar bone loss in ligature-induced periodontitis in rats. Int J Oral Biol. 2019;44(2):55-61.
[https://doi.org/10.11620/IJOB.2019.44.2.55]

-
García AV, Martínez MI, Landete MP, Moya MS, Sanahuja AB. Potential of Industrial Pineapple (Ananas comosus (L.) Merrill) By-Products as Aromatic and Antioxidant Sources. Antioxidants. 2021;10(11):1767.
[https://doi.org/10.3390/antiox10111767]

-
Ayoub L, Hassan F, Hamid S, Abdelhamid Z, Souad A. Phytochemical screening, antioxidant activity and inhibitory potential of Ficus carica and Olea europaea leaves. Bioinformation. 2019;15(3):226–32.
[https://doi.org/10.6026/97320630015226]

-
Mawa S, Husain K, Jantan I. Ficus carica L. (Moraceae): Phytochemistry, Traditional Uses and Biological Activities. Evid Based Complement Alternat Med. 2013;2013:974256.
[https://doi.org/10.1155/2013/974256]

-
Alim A, Li T, Nisar T, Ren D, Zhai X, Pang Y, Yang X. Antioxidant, antimicrobial, and antiproliferative activity-based comparative study of peel and flesh polyphenols from Actinidia chinensis. Food Nutr Res. 2019;63:1577.
[https://doi.org/10.29219/fnr.v63.1577]

-
Popovic M, Andjelkovic U, Grozdanovic M, Aleksic I, Gavrovic-Jankulovic M. In Vitro Antibacterial Activity of Cysteine Protease Inhibitor from Kiwifruit (Actinidia deliciosa). Indian J Microbiol. 2013;53(1):100-5.
[https://doi.org/10.1007/s12088-012-0319-2]

-
An X, Lee SG, Kang H, Heo HJ, Cho YS, Kim DO. Antioxidant and Anti-Inflammatory Effects of Various Cultivars of Kiwi Berry (Actinidia arguta) on Lipopolysaccharide-Stimulated RAW 264.7 Cells. J Microbiol Biotechnol. 2016;26(8):1367-74.
[https://doi.org/10.4014/jmb.1603.03009]

-
Kanzaki H, Wada S, Narimiya T, Yamaguchi Y, Katsumata Y, Itohiya K, Fukaya S, Miyamoto Y, Nakamura Y. Pathways that Regulate ROS Scavenging Enzymes, and Their Role in Defense Against Tissue Destruction in Periodontitis. Front Physiol. 2017;8:351.
[https://doi.org/10.3389/fphys.2017.00351]

-
Smida I, Pentelescu C, Pentelescu O, Sweidan A, Oliviero N, Meuric V, Martin B, Colceriu L, Bonnaure-Mallet M, Tamanai-Shacoori Z. Benefits of sea buckthorn (Hippophae rhamnoides) pulp oil-based mouthwash on oral health. J Appl Microbiol. 2019;126(5):1594-605.
[https://doi.org/10.1111/jam.14210]

-
Bueno-Silva B, Kawamoto D, Ando-Suguimoto ES, Alencar SM, Rosalen PL, Mayer MP. Brazilian Red Propolis Attenuates Inflammatory Signaling Cascade in LPS-Activated Macrophages. PLoS One. 2015;10(12): e0144954.
[https://doi.org/10.1371/journal.pone.0144954]

-
Szliszka E, Kucharska AZ, Sokół-Łętowska A, Mertas A, Czuba ZP, Król W. Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages. Evid Based Complement Alternat Med. 2013;2013:976415.
[https://doi.org/10.1155/2013/976415]

- Praveen NC, Rajesh A, Madan M, Chaurasia VR, Hiremath NV, Sharma AM. In vitro Evaluation of Antibacterial Efficacy of Pineapple Extract (Bromelain) on Periodontal Pathogens. J Int Oral Health. 2014;6(5):96-8.
-
Pavan R, Jain S, Shraddha, Kumar A. Properties and therapeutic application of bromelain: a review. Biotechnol Res Int. 2012;2012:976203.
[https://doi.org/10.1155/2012/976203]

-
Dini I, Falanga D, Di Lorenzo R, Tito A, Carotenuto G, Zappelli C, Grumetto L, Sacchi A, Laneri S, Apone F. An Extract from Ficus carica Cell Cultures Works as an Anti-Stress Ingredient for the Skin. Antioxidants (Basel). 2021;10(4):515.
[https://doi.org/10.3390/antiox10040515]

-
Deng J, Liu Q, Zhang C, Cao W, Fan D, Yang H. Extraction Optimization of Polyphenols from Waste Kiwi Fruit Seeds (Actinidia chinensis Planch.) and Evaluation of Its Antioxidant and Anti-Inflammatory Properties. Molecules. 2016;21(7):832.
[https://doi.org/10.3390/molecules21070832]

-
Iwasawa H, Morita E, Yui S, Yamazaki M. Anti-oxidant effects of kiwi fruit in vitro and in vivo. Biol Pharm Bull. 2011;34(1):128-34.
[https://doi.org/10.1248/bpb.34.128]