Influence of Ethanol Pretreatment on the Bonding of Resin Composite to Bleached Dentin
초록
본 연구에서는 근관치료된 변색치의 미백치료 후 복합레진 수복 시 미백제에 의한 부작용을 줄이기 위하여 사용되는 에탄올 전처리가 미백된 상아질과 복합레진의 접착에 미치는 영향을 평가하고자 사람의 소구치로 상아질 시편 제작 후, sodium perborate와 증류수의 혼합물을 7일간 적용하여 미백과정을 시행하였다. 미백 후 시편을 세 군으로 나누어 미백 직후, 7일간 증류수에 보관 후, 치면을 75% 에탄올 용액으로 3분간 처리 후 각각 접착술식을 시행하였다. 시편을 37℃에서 24시간 동안 물에 담근 후 미세인장결합강도를 측정하고, 파절면을 주사전자현미경을 이용하여 관찰하였다. 결합강도에 있어서, 에탄올로 전처리 또는 7일간 증류수에 보관한 경우 미백처리 하지 않은 대조군과 유의차를 보이지 않았으나 미백 직후 접착한 경우에는 대조군보다 유의하게 낮았다. 파절양상은 혼합성 파절이 우세하게 나타났으나 미백 직후 접착한 군에서는 주로 접착성 파절이 나타났다. 파절면의 현미경 관찰 시 에탄올로 처리한 군에서 대조군과 유사한 접착계면의 양상을 보였는데 균일한 두께의 혼성층과 길고 굵은 resin tag이 관찰되었다. 본 연구의 결과 미백 후 에탄올을 이용한 치면처리가 미백된 상아질에 대한 복합레진의 결합강도를 유의하게 증가시키는 것으로 보였다.
Keywords:
Bleached Dentin, Bond Strength, Ethanol Pretreatment, Nonvital BleachingⅠ. INTRODUCTION
Nonvital tooth bleaching has been considered as an effective and conservative esthetic treatment for endodontically-treated discolored teeth. Since adhesive restoration of access cavity usually follows bleaching, bonding between tooth and restoration material is important to long-term success of endodontic therapy and bleaching treatment (Howell, 1981). However, application of sodium perborate and/or hydrogen peroxide in nonvital bleaching may interfere with resin infiltration into etched enamel/dentin or inhibit polymerization of resins, leading to compromised bonding to bleached tooth surface (Teixeira et al., 2002; Timpawat et al., 2005).
A considerable reduction in bond strength of resin composite to tooth immediately after bleaching may occur because of the remnant of peroxide in the collagen matrix and dentinal tubules or change in organic content due to protein denaturation (Nour El-din et al., 2006; Bittencourt et al., 2010). Hence, delayed bonding after bleaching is commonly recommended to avoid problems related to decreased bonding. However, from the clinical standpoint, immediate restoration after nonvital bleaching is preferred in completing the restoration of the access cavity to prevent coronal leakage (Khoroushi et al., 2009).
It has been reported that application of ethanol solution, a drying agent to bleached enamel surface prior to bonding significantly reduced the adverse effect of bleaching on the bond strength of resin composite to enamel through its ability to extract water from the hard tissue (Barghi, 1994). Three-minute treatment with 70% ethanol seems to be an effective and fast-acting adjunct when the bonding procedure is carried out immediately (Kum et al., 2003; Niat et al., 2012). Notwithstanding the proven efficacy of ethanol pretreatment on composite-enamel bonding, relatively little attention has been paid to the effect of ethanol on the access cavity of intracoronally-bleached tooth in which the most part of bonding surface is dentin. Bonding to dentin has proved to be challenging because of the complex histological structure and variable composition of dentin itself (Zhang et al., 2014).
Therefore, this in vitro study investigated the effect of ethanol pretreatment following nonvital bleaching on composite bonding to dentin. The null hypothesis tested was that the application of ethanol solution on bleached dentin surface prior to bonding would not affect the microtensile bond strength of resin composite to dentin.
Ⅱ. MATERIALS AND METHODS
1. Tooth selection and specimen preparation
Sixteen human maxillary premolars were collected with patients' informed consents and the study was approved by the Institutional Review Board (IRB) of Kyungpook National University Hospital, Daegu, Korea (document number: BMRI 74005-452).
The teeth were cleaned, disinfected in 0.1% thymol solution for 7 d, and stored in distilled water at 4℃ until use. The occlusal enamel and roots of teeth were removed using a low-speed diamond saw (Isomet, Buehler Ltd., Lake Bluff, IL, USA) under water-cooling to form 5-6 mm thick, parallel-sided crown segments. The exposed tooth surface was further wet-polished with a 600-grit silicon carbide paper to create a flat, midcoronal dentin surface (Spyrides et al., 2000).
The specimens were randomly divided into four groups of four specimens each. All specimens except for the control group (no bleaching) were subjected to a bleaching procedure by applying a mixture of sodium perborate (Junsei Chemical Co., Ltd., Tokyo, Japan) and distilled water in a 1:1 ratio onto the dentin surface for 7 d, capped with the customized tray to prevent loss of the bleaching agent from the tooth surface during the storage in 100% relative humidity at 37℃ (Elkhatib et al., 2003).
The dentin surfaces of group IB (immediate bonding) were restored immediately after bleaching using the bonding procedure. For group DB (delayed bonding), the teeth were immersed in distilled water for 7 d and subjected to bonding. For group ET (ethanol treatment), 75% ethanol solution was applied onto the bleached dentin surface for 3 min (Table 1).
In the bonding steps, all tooth surfaces were etched with 32% phosphoric acid (Uni-Etch, Bisco Inc., Schaumburg, IL, USA) for 15 s. After rinsing and drying, adhesive (One-Step, Bisco Inc.) was applied to the etched surface according to the manufacturer's instructions and light-cured for 10 s with a LED light-curing unit (Bluephase, Ivoclar Vivadent Inc., Amherst, NY, USA; 1000 mW/cm2 irradiance). Resin composite (AELITETM All-purpose body, A2 shade, Bisco Inc.) was built up in three 2-mm increments. Each composite layer was light-cured for 20 s and final cure was done for 40 s. All teeth of four groups were stored in distilled water at 37℃ for 24 h before sectioning.
2. Microtensile bond strength testing and failure mode analysis
Each bonded specimen was longitudinally sectioned into 1 mm-thick slabs with the low-speed diamond saw (Isomet, Buehler Ltd.). Each slab was fixed on a glass platform with sticky wax and serially sectioned into 1 mm2 (± 0.1 mm2) sticks, in accordance with the ‘non-trimming’ version of the microtensile test (Shono et al., 1999). The exact dimensions of each stick were measured using a digital caliper to calculate the precise cross-sectional area.
For microtensile bond strength testing, five sticks were sectioned from the center of each bonded specimen (Hiraishi et al., 2009). Thus, the use of four teeth resulted in a total of 20 sticks for each of the groups tested. The bonded composite-dentin sticks were attached to a testing device with cyanoacrylate glue (Zap-It, DVA, Corona, CA, USA). The device was attached to a microtensile bond tester (Bisco Inc.) and loaded in tension at a crosshead speed of 1 mm/min until failure. Premature failures were not considered in the data analysis (Briso et al., 2014).
After fracturing, all specimens were examined under the stereomicroscope (SZ4045, Olympus, Tokyo, Japan) at a magnification of 25× and failure modes were classified into one of following modes: (a) adhesive failure between dentin and the adhesive resin layer; (b) cohesive failure in dentin; (c) cohesive failure in resin composite; (d) mixed failure involving the combinations of cohesive failures in adhesive, composite or dentin. Each type of failure mode was expressed as a percentage of the total number of specimens in that group.
3. Examination of the fractured surfaces using scanning electron microscopy (SEM)
To observe the hybrid layer of bonded specimens, representative fractured sticks (dentin sides, n = 2) were selected from each group with tensile bond strengths that were close to the mean bond strength of that group. Bonded interfaces were polished using No. 400-, No. 600-, and No. 1,200-grades of silicon carbide papers (3M, St. Paul, MN, USA). After sonication in distilled water for 5 min, specimens were demineralized in 1N HCl for 5 min, deproteinized in 5.25% NaOCl for 5 min. and then sequentially dehydrated in ascending grades of ethanol (25%, 50%, 75%, and 95% for 20 min each, and 100% for 60 min) (Ferreira et al., 2011). After a 24 h air drying, the specimens were sputter-coated with gold and examined under a field emission-scanning electron microscope (FE-SEM, SU8220, Hitachi, Tokyo, Japan).
4. Statistical analysis
The normally distributed (as determined by the Kolmogorov-Smirnov test) bond strength was analyzed with one-way analysis of variance (ANOVA) followed by multiple comparisons with Tukey’s test at the 0.05 significance level. The statistical analysis was performed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA).
Ⅲ. RESULTS
Table 2 summarizes microtensile bond strengths of composite resin to bleached dentin surface treated with different regimens prior to bonding. One-way ANOVA showed statistically significant differences among the test groups (p < 0.001). Group IB showed a significantly lower microtensile bond strength than the control group (p < 0.001) and the groups DB and ET statistically similar values to the control group (p > 0.05).
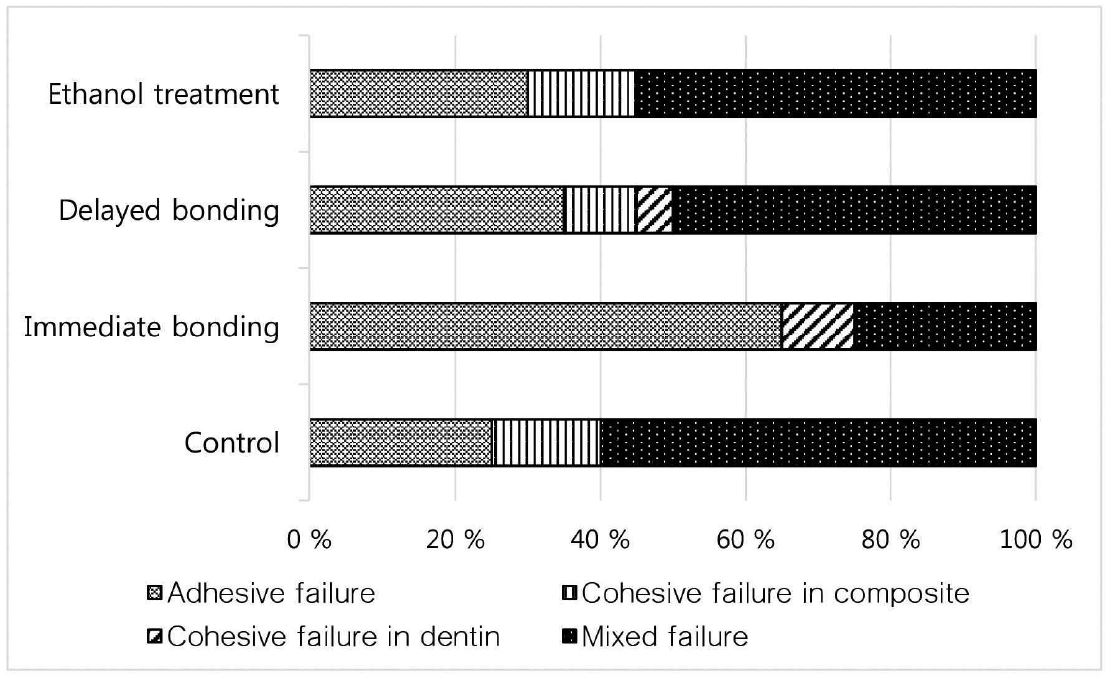
Figure 1 shows the failure pattern distribution (%) as assigned using the light microscope. Mixed failure mode was predominant in all groups except for in the IB group which presented a high incidence of adhesive failure.
The representative SEM micrographs showing resin penetration in the fractured dentin sides are presented in Figure 2. Long, dense resin tags as well as a uniform hybrid layer were seen in the control (non-bleached) specimen (Figure 2A). In the specimen from the IB group, resin tags were sparse, short and disorganized. A hybrid layer was barely observed (Figure 2B). Delayed bonding showed a thin, uniform hybrid layer and dense, thick and short resin tags (Figure 2C). Ethanol treatment resulted in a compact and homogenous hybrid layer with long, thick and dense resin tags (Figure 2D).
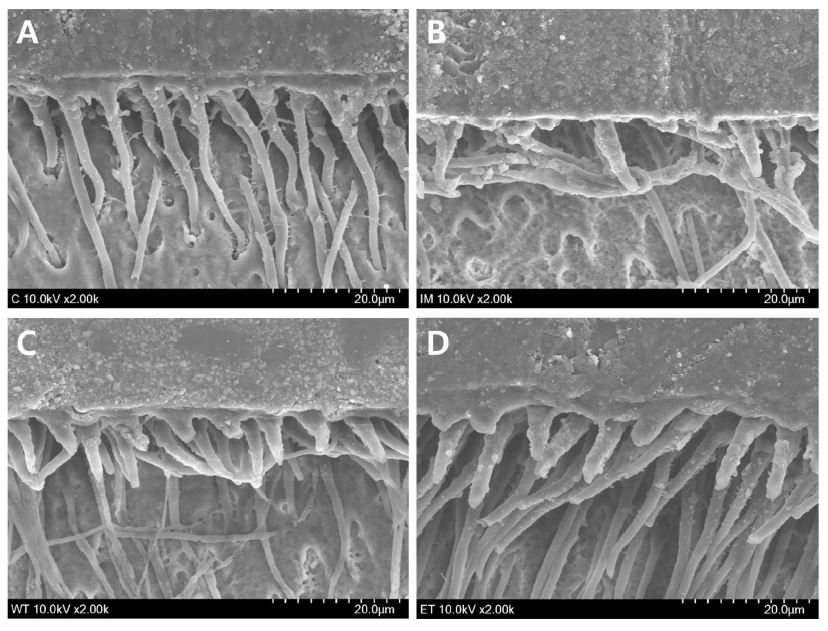
Representative SEM micrographs showing hybrid layer and resin penetration in the fractured dentin sides. (A) non-bleached control; (B) immediate bonding; (C) delayed bonding; (D) ethanol treatment (2000×).
Ⅳ. DISCUSSION
The present in vitro study confirmed that immediate bonding after nonvital bleaching produces a significant decrease in bond strength of resin composite to dentin surface. In contrast, when the composite bonding was delayed until 7 days after bleaching, the bond strength increased up to the value obtained for the non-bleached control group. 3-min application of 75% ethanol solution on the dentin surface prior to bonding resulted in a complete reversal of the compromised bond strength in the bleached dentin. Therefore, the null hypothesis that the application of ethanol solution would not affect composite bonding to bleached dentin was rejected.
The typical method for avoiding problems with bonding to bleached teeth is simply to delay bonding procedures. Because the residual oxygen after bleaching slowly dissipates over time, a certain waiting period is required for a gradual elimination of oxygen from the bleached surface before adhesive restoration (Torneck et al., 1990; Teixeirare et al. 2002). There are remarkable variations among the recommended post-bleaching time and some authors thought that a delay of at least 2 weeks is necessary for regaining the pre-bleaching level in adhesion (Shinohara et al., 2005; Barbosa et al., 2008). Because the effect of bleaching might be more permanent or take longer to eliminate in dentin due to MMP-mediated collagen degradation in dentin-resin bonded interfaces (Toledano et al., 2011), up to 4 weeks may be needed to delay bonding procedures for reversal of decreased bonding efficacy. However, from the finding of this study, one week seems to be long enough for optimal bonding since peroxide completely leaches from bleached dentin surface within seven days after its application (Adibfar et al., 1992). In addition, the storage medium, water also acts to dilute the effect of residual oxygen from the bleaching agent during the waiting period (Torneck et al., 1991). Although the hybrid layer and resin tags formed in the delayed bonding group was thinner and shorter than those in the unbleached and ethanol treatment groups, the bond strengths were not significantly different among the three groups. This finding supports that an important feature for bond strength is the quality of the formed hybrid layer, not the thickness of hybrid layer and resin tag length (Rahal et al., 2011).
Sodium perborate is a white, water-soluble chemical compound with the chemical composition NaBO3. It is generally used as the form of sodium perborate-water paste in the “walking bleaching” technique (Freccia et al., 1982; Timpawat et al., 2005). The decomposition reaction of sodium perborate is slow and releases hydrogen peroxide (approximately 9%) in a low concentration, consequently releasing water and oxygen free radicals. Reduction in bond strength in bleached tooth surface is believed to be caused by residual oxygen present in enamel and dentin pores after completion of bleaching (Demarco et al., 1998). The use of acetone-based adhesive systems may eliminate the adverse effects of bleaching on bonding to teeth with no need of elapsed time following bleaching due to its water-chasing behavior (Barghi, 1994). Acetone in the
bonding agent can displace the surface water containing residual oxygen from bleaching agents. According to Spyrides et al. (2000), the water and oxygen chasing effect of acetone and ethanol can be intensified in dentin because of its intrinsic moisture. However, in the present study, the immediate bonding led to a considerable reduction in bond strength despite using the acetone based adhesive, One-Step for bonding. The failure mode analysis also showed that the immediate bonding primarily failed at the adhesive interface, whereas mixed failure was predominant in the other groups, control, DB and ET. Under the SEM, resin tags in bleached dentin subsequently undertaken the bonding procedure were less defined, more fragmented, and less penetrated than in the unbleached control. These findings suggest that the acetone in the adhesive failed to displace water properly and the residual oxygen might interfere with resin attachment, infiltration and polymerization, producing the poorly uniformed and organized interface (Uysal et al., 2009).
Meanwhile, the application of ethanol on the bleached dentin surface allowed earlier composite restoration with improved adhesion in this study. The complete reversal of reduced bond strength was attained with the use of the 75% ethanol solution for 3 min before bonding. In terms of failure mode, ethanol application showed the predominant mixed failure compared with the immediate bonding, indicating a better clinical bond in the former than in the latter. The bonding interface showed a uniform hybrid layer with well defined and deeply penetrated resin tags, which is similar to that formed in the unbleached control. Alcohol has been known to be able to significantly reduce the detrimental effects of bleaching on compositeenamel bond as a drying agent (Barghi et al., 1994). In this study, unlike acetone, removal of the oxygen by alcohol appears to help resin infiltrate into dentin and polymerize in situ by preventing oxygen-inhibited polymerization (Sadek et al., 2010), resulting in the strong interface able to withstand debonding forces sufficiently. Taking it into account, the effect of adhesive systems on composite bond strength to bleached teeth seems to depend on the solvents used and alcohol might be responsible for eliminating the detrimental effects of residual oxygen by the interaction with oxygen (Kalili et al, 1991).
The microtensile bond strength test was used in this study to evaluate composite bonding to bleached dentin. Unlike the shear and conventional tensile bond tests, which have been commonly used for the purpose, the microtensile test allows the testing of very small cross-sectional areas of dentine-resin specimens (1.0 ± 0.2 mm2) (Shono et al., 1999) and develops a uniform stress distribution during loading (Pashley et al., 1995). However, the failure of a certain number of microtensile specimens during their preparation and before loading is a common and undesirable occurrence (Ferrari et al., 2002). While preparing specimens, microcracks might be inadvertently produced mainly by the vibrations of cutting instruments. Such defect is responsible for the premature failure of bonded specimens. In the present study, the frequency of premature failures in all test groups was similar. Hence, pretest failures were ignored and not used in the statistical analysis. Moreover, to minimize the regional differences in the bond strength (Pereira et al., 1999), slabs sectioned from the center of each composite block-bonded crown segment were selected. Beams derived from the periphery of each slab were not used for bond testing.
To dissolve remnants of peroxide after nonvital bleaching, access cavities can also be cleaned with catalase or antioxidant (Rostein, 1993; Moosavi et al., 2010). However, the use of these agents to neutralize the oxidizing effect of bleaching agent appears to be time-consuming or expensive (Park et al, 2013). Ethanol has some advantages clinically over the neutralizing agents. 70% ethanol is generally used for disinfection and sterilization in healthcare facilities. It is easily available in the clinic and has no need of special storage. Therefore, using ethanol solution after bleaching for immediate bonding is more feasible when it is difficult to delay resin composite restoration for one week. In the present study, 75% ethanol solution available for daily use in our clinic was applied on the bleached dentin surface and bond strength increased up to the level obtained in the non-bleached control. The finding of this in vitro study suggests that 3-min application of 75% ethanol solution to intracoronally-bleached dentin by applying a mixture of sodium perborate and water for 7 d is needed to address the impaired composite bonding. The efficiency of ethanol pretreatment on retaining the longevity of resin-dentin bonds after nonvital bleaching should be elucidated in further studies.
Ⅴ. CONCLUSION
Within the limitations of this study, it was found that dentin surface pretreatment with ethanol prior to bonding procedures resulted in a complete reversal of the compromised microtensile bond strength in bleached dentin. Application of 75% ethanol solution for 3 min onto dentin surface after nonvital bleaching may be effective in eliminating the adverse effect of bleaching on compositedentin bonding.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2012382).
Ⅵ. REFERENCES
-
Adibfar, A., Steele, A., Torneck, CD., Titley , KC., Ruse, D., (1992), Leaching of hydrogen peroxide from bleached bovine enamel, J Endod, 18, p488-491.
[https://doi.org/10.1016/S0099-2399(06)81348-8]

- Barbosa, CM., Sasaki, RT., Florio, FM., Basting, RT., (2008), Influence of time on bond strength after bleaching with 35% hydrogen peroxide, J Contemp Dent Pract, 9, p81-88.
-
Barghi, N., Godwin, JM., (1994), Reducing the adverse effect of bleaching on composite-enamel bond, J Esthet Dent, 6, p157-161.
[https://doi.org/10.1111/j.1708-8240.1994.tb00852.x]

-
Bittencourt, ME., Trentin, MS., Linden, MSS., Arsati, YB., Franca, FMG., Flόrio, FM., Basting, RT., (2010), Influence of in situ postbleaching times on shear bond strength of resin-based composite restorations, J Am Dent Assoc, 141, p300-306.
[https://doi.org/10.14219/jada.archive.2010.0164]

-
Briso, AL., Rahal, V., Sundfeld, RH., dos Santos, PH., Alexandre, RS., (2014), Effect of sodium ascorbate on dentin bonding after two bleaching techniques, Oper Dent, 39, p195-203.
[https://doi.org/10.2341/12-054-L]

- Demarco, FF., Turbino, M., Jorge, AG., Matson, E., (1998), Influence of bleaching on dentin bond strength, Am J Dent, 11, p78-82.
- Elkhatib, H., Nakajima, M., Hiraishi, N., Kitasako, Y., Tagami, J., Nomura, S., (2003), Surface pH and bond strength of a self-etching primer/adhesive system to intracoronal dentin after application of hydrogen peroxide bleach with sodium perborate, Oper Dent, 28, p591-597.
-
Ferrari , M., Goracci , C., Sadek , F., Eduardo , P., Cardoso , C., (2002), Microtensile bond strength tests: scanning electron microscopy evaluation of sample integrity before testing, Eur J Oral Sci, 110, p385-391.
[https://doi.org/10.1034/j.1600-0722.2002.21317.x]

-
Ferreira, EA., Souza-Gabriel, AE., Silva-Sousa, YC., Sousa-Neto, MD., Silva, RG., (2011), Shear bond strength and ultrastructural interface analysis of different adhesive systems to bleached dentin, Microsc Res Tec, 74, p244-250.
[https://doi.org/10.1002/jemt.20895]

-
Freccia, WF., Peters, DD., Lorton, L., Bernier, WE., (1982), An in vitro comparison of nonvital bleaching techniques in the discolored tooth, J Endod, 8, p70-77.
[https://doi.org/10.1016/S0099-2399(82)80261-6]

- Hiraishi, N., Yiu, CK., King, NM., Tay, FR., (2009), Effect of pulpal pressure on the microtensile bond strength of luting resin cements to human dentin, Dent Mater, 25(58), -66.
-
Howell, RA., (1981), The prognosis of bleached root-filled teeth, Int Endod J, 14, p22-26.
[https://doi.org/10.1111/j.1365-2591.1981.tb01055.x]

- Kalili, T., Caputo, AA., Mito, R., Sperbeck, G., Matyas, J., (1991), In vitro toothbrush abrasion and bond strength of bleached enamel, Pract Periodontics Aesthet Dent, 3, p22-24.
- Khoroushi, M., Feiz, A., Ebadi, M., (2009), Influence of intermediary filling material on microleakage of intracoronally bleached and restored teeth, Dent Res J (Isfahan), 6, p17-22.
- Kum, KY., Lim, KR., Lee, CY., Park, KH., Safavi, KE., Fouad, AF., Spangberg, LS., (2003), Effect of removing residual peroxide and other oxygen radicals on the shear bond strength and failure modes at resin-tooth interface after tooth bleaching, Am J Dent, 16, p267-270.
- Moosavi, H., Moghaddas, MJ., Ghoddusi, J., Rajabi, O., (2010), Effects of two antioxidants on the microleakage of resin-based composite restorations after nonvital bleaching, J Contemp Dent Pract, 11, pE033-40.
- Niat, AB., Yazdi, FM., Koohestanian, N., (2012), Effects of drying agents on bond strength of etch-and-rinse adhesive systems to enamel immediately after bleaching, J Adhes Dent, (14), p511-516.
-
Nour El-din, AK., Miller, BH., Griggs, JA., Wakefield, C., (2006), Immediate bonding to bleached enamel, Oper Dent, 31, p106-114.
[https://doi.org/10.2341/04-201]

-
Park, JY., Kwon, TY., Kim, YK., (2013), Effective application duration of sodium ascorbate antioxidant in reducing microleakage of bonded composite restoration in intracoronally-bleached teeth, Restor Dent Endod, 38, p43-47.
[https://doi.org/10.5395/rde.2013.38.1.43]

-
Pashley, DH., Sano, H., Ciucchi, B., Yoshiyama, M., Carvalho, RM., (1995), Adhesion testing of dentin bonding agents: A review, Dent Mater, 11, p117-125.
[https://doi.org/10.1016/0109-5641(95)80046-8]

-
Pereira, PN., Okuda, M., Sano, H., Yoshikawa, T., Burrow, MF., Tagami, J., (1999), Effect of intrinsic wetness and regional difference on dentin bond strength, Dent Mater, 15, p46-53.
[https://doi.org/10.1016/S0109-5641(99)00013-5]

- Rahal, V., Briso, AL., dos Santos, PH., Sundefeld, ML., Sundfeld, RH., (2011), Influence of the hybrid layer thickness and resin tag length on microtensile bond strength, Acta Odontol Latinoam, 24, p8-14.
-
Rotstein, I., (1993), Role of catalase in the elimination of residual hydrogen peroxide following tooth bleaching, J Endod, 19, p567-569.
[https://doi.org/10.1016/S0099-2399(06)81288-4]

-
Sadek, FT., Braga, RR., Muench, A., Liu, Y., Pashely, DH., Tay, FR., (2010), Ethanol wet-bonding challenges current anti-degradation strategy, J Dent Res, 89, p1499-1504.
[https://doi.org/10.1177/0022034510385240]

-
Shinohara, MS., Peris, Ar., Pimenta, LA., Ambrosano, GM., (2005), Shear bond strength evaluation of composite resin on enamel and dentin after nonvital bleaching, J Esthet Restor Dent, 17, p22-29.
[https://doi.org/10.1111/j.1708-8240.2005.tb00078.x]

-
Shono, Y., Ogawa, T., Terashita, M., Carbalho, RM., Pashley, EL., Pashley, DH., (1999), Regional measurement of resin-dentin bonding as an array, J Dent Res, 78, p699-705.
[https://doi.org/10.1177/00220345990780021001]

-
Spyrides, GM., Perdigão, J., Pagani, C., Araújo, MA., Spyrides, SM., (2000), Effect of whitening agents on dentin bonding, J Esthet Dent, 12, p264-270.
[https://doi.org/10.1111/j.1708-8240.2000.tb00233.x]

- Teixeira, ECN., Hara, AT., Turssi, CP., Serra, MC., (2002), Effect of nonvital tooth bleaching on resin/enamel shear bond strength, J Adhes Dent, 4, p317-322.
-
Timpawat, S., Nipattamanon, C., Kijsamanmith, K., Messer, HH., (2005), Effect of bleaching agents on bonding to pulp chamber dentin, Int Endod J, 38, p211-217.
[https://doi.org/10.1111/j.1365-2591.2004.00931.x]

-
Toledano, M., Yamauti, M., Osorio, E., Osorio, R., (2011), Bleaching agents increase metalloproteinases-mediated collagen degradation in dentin, J Endod, 37, p1668-1672.
[https://doi.org/10.1016/j.joen.2011.08.003]

-
Torneck, CD., Titley, KC., Smith, DC., Adibfar, A., (1990), Adhesion of light-cured composite resin to bleached and unbleached bovine dentin, Endod Dent Traumatol, 6, p97-103.
[https://doi.org/10.1111/j.1600-9657.1990.tb00401.x]

-
Torneck, CD., Titley, KC., Smith, DO., Adibfar, A., (1991), Effect of water leaching the adhesion of composite resin to bleached and unbleached bovine enamel, J Endod, 17, p156-160.
[https://doi.org/10.1016/S0099-2399(06)82008-X]

-
Uysal, T., Er, O., Sagsen, B., Ustdal, A., Akdogan, G., (2009), Can intracoronally bleached teeth be bonded safely?, Am J Orthod Dentofacial Orthop, 136, p689-694.
[https://doi.org/10.1016/j.ajodo.2007.11.033]

-
Zhang, L., Wang, DY., Fan, J., Li, F., Chen, YJ., Chen, JH., (2014), Stability of bonds made to superficial vs. deep dentin, before and after thermocycling, Dent Mater, 30, p1245-1251.
[https://doi.org/10.1016/j.dental.2014.08.362]